Physical Properties of Matter
advertisement

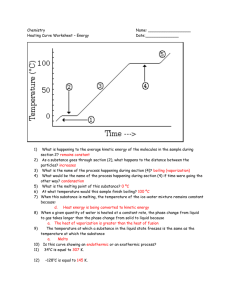
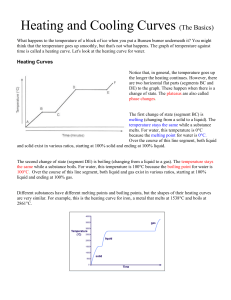
Physical Properties of Matter Review Mixtures may be separated by physical means (particle size, boiling pt. density etc) Phases of matter Solid – particles close, regular pattern definite shape and volume Liquid – particles close no regular pattern, no definite shape definite volume Gases – particles spread no definite shape or volume Energy – exothermic energy given off/ endothermic energy absorbed Energy measured in joules Heat always flows from hot to cold Measuring energy during change of temperature q=mct c water is 4.18j/g Measuring energy change during fusion (melting) q=mhf hf water is 334j/g Measuring energy change during vaporization (boiling) q=mhv hv water is 2260j/g Temperature = average kinetic energy Kelvin temperature = Celsius + 273 one degree kelvin = one degree Celsius Heating/Cooling Curves Plateaus are phase changes (melting and boiling in heating curve, condensation and freezing in cooling curve) Potential energy rising in heating curve falling in cooling curve. Kinetic energy stays the same Diagonals are phases of matter. Kinetic energy rising in heating curve falling in cooling curve. Potential energy stays the same. Solid to Liquid (melting fusion) and Liquid to Gas(vaporization, boiling) are endothermic Gas to Liquid (condensation) and Liquid to Solid (freezing) are exothermic Sublimation Solid to Gas (carbon dioxide and Iodine) Kinetic Molecular Theory (Ideal Gas) Gases particle size negligible, Particles move randomly in straight lines, No attractive forces between particles, No energy (elastic) Gases most Ideal in High Temperature/Low Pressure Hydrogen and Helium most ideal Boyles law (inversely proportional) P1V1 =P2V2 (constant temperature) Charles law (directly proportional) V1/T1 = V2/T2 (constant pressure) temp must be in Kelvin. Together combined gas law PV/T =PV/T temp must be in Kelvin Standard temp and pressure 0C or 273K and 1atm or 101.3Kpa