Hydrat I CuSO4
advertisement

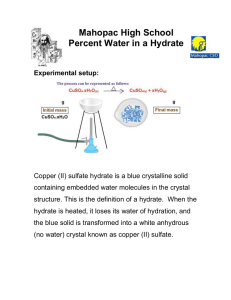
Hydrate Lab Title: Percent of Water in a Hydrate Objective: Compare the experimental to the calculated percent Chemical: 2 grams Copper Sulfate Materials: Data: Mass of Empty Crucible: Mass of Crucible and Hydrate: Mass of Crucible and Anhydrous Salt: Analysis Questions: 1. The true value of the percentage of water in this hydrate is 36%. What is your percent error? 2. Why must you allow the crucible to cool before measuring the mass? 3. Why must you measure the mass of the anhydrous salt immediately upon cooling? 4. Given the true mole masses of CuSO4 (160g) and H2O (18g), how could you find the exact formula of the hydrate from your experimental data? How could you find the exact value of x in CuSO4•xH2O? 5. Why was the crucible inspected prior to the lab? 6. Why was the anhydrous salt reheated if time allowed?