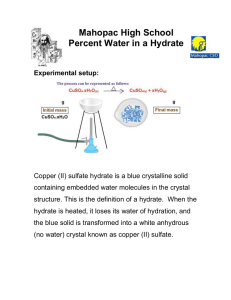
Formula of a Hydrate Lab Safety: Crucibles are VERY HOT; always handle them with tongs. DO NOT put hot crucibles on a balance! Crucibles are VERY FRAGILE. Wear goggles at all times. Introduction: A hydrate is a chemical that has water molecules loosely bonded to it. The water molecules are not actually part of the formula, so the formula is written slightly differently. An example would be CaSO4 . 3H2O. This chemical would be called calcium sulfate trihydrate. The formula indicates that there are three moles of water in the crystal for every mole of calcium sulfate present. When finding the mass of this chemical, you find the mass of the calcium sulfate and then add 3 times the mass of water to it. (40.08 + 32.066 + 4(15.999) + 3(2(1.0079) + 15.999)) = 190.19 g/mol. The water can easily be removed from a hydrate just by heating strongly. You will be massing a hydrate and heating it to remove the water, producing what is now called an "anhydrous salt”(without water), and weighing it again. You can now find the percent of the anhydrous salt and the water. By finding a mole ratio, you can find out how many moles of water were removed per mole of anhydrous salt. This number goes just before the H2O in the formula. Often removing the water will affect the crystalline structure. Interesting things happen when the water is added back to the crystal. You will be using the hydrate CuSO4 . ?H2O. The purpose of this lab is to determine the formula of this hydrate by heating and weighing it to a constant mass. The hypothesis for the lab is: If the mass lost by a hydrate after heating is measured, then the formula of the hydrate can be determined. Sample CalculationAn empty crucible has a mass of 12.77 grams. The crucible and hydrate have a mass of 13.45 grams. After heating, the crucible and anhydrous salt have a mass of 13.01 grams. What is the formula of this hydrate of magnesium sulfate: MgSO4 . ??H2O Mass of hydrate = 13.45 - 12.77 = 0.68 grams Mass of anhydrous salt = 13.01 - 12.77 = 0.24 grams Mass of water = 13.45 - 13.01 = 0.44 grams Percent by mass of water in the hydrate = 0.44 grams/0.68 grams x 100 = 65% water Moles of anhydrous salt = 0.24 grams MgSO4 x ___1 mol MgSO4__________ = .0020 moles MgSO4 120.367 g MgSO4 Moles of water = 0.44 grams H2O x _______1 mol H2O_________ = 0.024 moles H2O 18.0148 g H2O Ratio of moles of water to moles of anhydrous salt = Therefore the formula is: Prelab: 1. Explain why you must repeatedly heat and weigh and heat and weigh the anhydrous salt until the mass doesn’t change anymore. 2. a. Considering the procedure for the lab, does breaking the bonds between the copper sulfate and the attached water molecules require energy or release energy? b. Considering your answer to part a, do you think that re-forming the bond between the anhydrous salt and water molecules would require energy or release energy? Procedure: 1. Find the mass of a clean, dry crucible and lid on an accurate balance. Record in data table. 2. Obtain a scoop of the hydrate from your teacher into your crucible and find the mass again. Record in data table. 3. Carefully take your crucible to a hot plate and begin heating slowly, uncovered. Increase the heat until you have heated the crucible strongly for about 10 minutes. 4. Remove the crucible from the hot plate. Let it cool on the counter, covered, for a few minutes and then carefully carry to the balance. 5. Find the mass of the anhydrous crystal, crucible, and cover. Record in data table. 6. Repeat heating strongly for another two minutes, cool, and measure mass again. Record in data table. If it is different, then continue this process until you reach a constant mass (within 0.05 grams). Record in data table. 7. Write down a hypothesis for what you think will happen if you add water back into the anhydrous salt. Test your hypothesis and record your observations here (you should notice at least two of the eight observations that we discussed in the physical/chemical changes activity). Hypothesis: Observations: Calculations: 1) Find the experimental percent by mass of water in the hydrated crystal and record in your data table. Show your work here: 2) Find the moles of anhydrous crystal and the moles of water and record in your data table. Show your work here: 3) Use the ratio of anhydrous crystal to water to predict the formula for the hydrated crystal and record in your data table. Show your work here: 4) Ask me for the accepted formula and find the accepted percentage of water in the hydrated crystal. Show your work here: 5) Find the percent error in your results. (theoretical - experimental)/theoretical x 100 Record in your data table, and show your work here: Conclusion questions: 1) This method is not appropriate for determining the formula for all hydrates. Give at least one reason why it might not work. 2) Imagine that you are a research chemist working for a chemical company. Brainstorm at least one use of water of hydrate crystals in a laboratory setting. 3) Describe two sources of error that would make the calculated percent water in the hydrate incorrectly low, and explain why these would lower the apparent amount of water in the hydrate.. Then describe two sources of error that would make the calculated percent water in the hydrate incorrectly high, and explain why these would increase the apparent amount of water in the hydrate. 4) Explain both of the observations that you made when water was added to the anhydrous salt - in other words, why did they happen? 5) Explain why it does not violate the Law of Conservation of Matter that the anhydrous salt weighed less than the hydrate that you started with. DATA: Mass of crucible and cover: ___________ Mass of crucible/cover and hydrated crystal: ___________ Mass of hydrated crystal: _________ Mass of crucible/cover and anhydrous crystal: _________ __________ Mass of anhydrous crystal: ____________ Mass of water in the hydrate: __________ CALCULATION RESULTS: Percent water in the hydrate: __________ Moles of anhydrous crystal: ___________ Moles of water in the hydrate: _________ Formula of the hydrate: __________ Percent error: ____________ __________ ___________