work sheet 4
advertisement

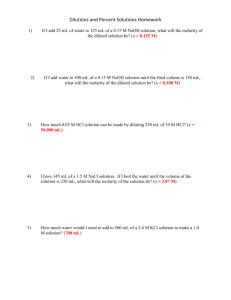
`المملكة العربية السعودية Kingdom of Saudi Arabia وزارة التعليم العالي Ministry of Higher جامعة المجمعة Education عمادة السنة التحضيرية Majma'a University Preparatory Year Deanship Work sheet (4) 1) Write the net ionic equation for the following reaction: AgNO3 (aq) + NaCl(aq) → NaNO3(aq) + AgCl(s) …………………………………………………………………………… …………………………………………………………………………… …………………………………………………………………………… …………………………………………………………………………… …………………………………………………………………………… K2CO3(aq) + CaI2(aq) → 2KI(aq) + CaCO3(s) …………………………………………………………………………… …………………………………………………………………………… …………………………………………………………………………… …………………………………………………………………………… …………………………………………………………………………… CuCl2 (aq) + 2NaOH(aq) → Cu(OH)2 (S) + 2NaCl(aq) …………………………………………………………………………… …………………………………………………………………………… …………………………………………………………………………… …………………………………………………………………………… ……………………………………………………………………………. 2) What is The molar mass of the following: 1- CH2Cl2 ……………………………………………………………………… 2- NH4Cl ............................................................................................................. 3- Na2CO3 ........................................................................................................... 3) Calculate the molarity of solution prepared by 5.00g of NaOH in volume 250 ml of solution? ……………………………………………………………………………… ……………………………………………………………………………… ……………………………………………………………………………… ……………………………………………………………………………… ……………………………………………………………………………… 4) Calculate the number of mole for 32.3ml of 0.625 M CaCl2 ……………………………………………………………………………… ……………………………………………………………………………… ……………………………………………………………………………… ……………………………………………………………………………… 5) A solution of 5 ml of HCl required 10 ml of 0.12 M NaOH solution for complete neutralization. What is the molarity of HCl solution? ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... .......................................................................................................................... 6) How many milliliters of water must be added to 150 ml of 3.00M KOH to give 1.25M solution? ……………………………………………………………………………… ……………………………………………………………………………… ……………………………………………………………………………… ……………………………………………………………………………… ……………………………………………………………………………… 7) Complete the following equation : 1- NH3 + H2O 2- HCLO4 + Ca(OH)2 3- HCl + NaOH 4- H2SO4 + KOH Atomic number: (C = 12 O =16 N=14 Na =23 H =1 CL= 35.5 g\mol)