Chemistry Part 1 Review
advertisement

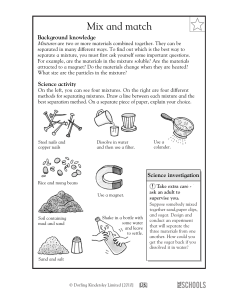
Name ____________________________________________ Atoms, Elements, Compounds and Mixtures Exam Review Part 1: Fill in the blanks for questions 1-24. 1. Matter consists of ____________________________________. 2. An _______________________________ is made up of only one type of atom. 3. A __________________________________ is made up of 2 or more different elements. 4. A compound consists of 2 or more DIFFERENT ELEMENTS _____________________________ combined. 5. The smallest part of a compound is an ___________________________________. 6. Protons have a ____________________ charge while electrons have a _________________________ charge. 7. Neutrons are ___________________________ because they have no charge. 8. The ___________________________________of an atom contains protons and neutrons. 9. ________________________________ are found outside the nucleus. 10. The atomic number represents the number of _____________________________ in an atom. 11. H2O is an example of a _________________________________ because it is made up of 2 different elements. 12. Carbon (C) is an example of an _______________________________ because it is made up of only one type of atom. 13. An _____________________________________ CANNOT be broken down. 14. A mixture consists of 2 or more different substances _________________________________ combined. 15. The substance that dissolves in a liquid is called the _______________________________________. 16. The substance that does the dissolving is called the ________________________________________. 17. A solution consists of a ______________________________ and a ________________________________. 18. Anything metallic can be separated from a mixture by using a ______________________________. 19. Rocks can be separated by sand using a _____________________________________. 20. ___________________________________ is used to separate pigments from a mixture. 21. Salt can be separated from a salt water solution by using _________________________________. 22. Sand can be separated from liquid mixture by using ___________________________ paper. 23. An _____________________________ substance CANNOT dissolve in a solvent while a _______________________________ CAN dissolve in a solvent. 24. Three factors that can increase the solubility of a substance is ______________________________________, ________________________________________ and ___________________________________. Part 2: Write you answers in the spaces provided. 25. Sand, sugar and water are mixed together in a beaker. a. Identify the solvent. _______________________________________________________ b. Identify the solute. Support your answer. __________________________________________________________________________________ c. Identify the soluble substance. ___________________________________________________________ d. Identify the insoluble substance. _________________________________________________________ e. What can be used to separate the sand from the sugar water solution? ___________________________________________________________________________________ f. How can the sugar be separated from the solution? ___________________________________________________________________________________ 26. Contrast an element and a compound. _______________________________________________________________________________________________ _______________________________________________________________________________________________ 27. Is carbon dioxide (CO2) a compound or an element? Support your answer. _______________________________________________________________________________________________ _______________________________________________________________________________________________ 28. Can carbon dioxide be broken down? Support your answer. _______________________________________________________________________________________________ _______________________________________________________________________________________________ 29. Compare and contrast a compound and a mixture. _______________________________________________________________________________________________ _______________________________________________________________________________________________ _______________________________________________________________________________________________ _______________________________________________________________________________________________