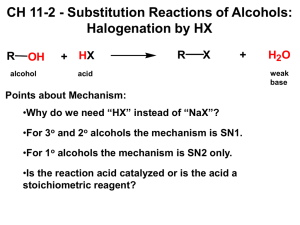ALCOHOLS
advertisement

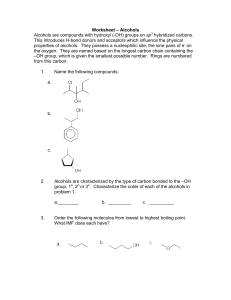
ALCOHOLS Corbin Frye, Taylor Galpern, Julie Ghekas Alcohols in Action… Alcoholic beverages Methanol and ethanol Industrial, medical Perfumes/essences Functional Group: Alcohols …a hydroxyl group (-OH) bound to a single carbon atom. General Formula: CnH2n+1OH Identification of Alcohols Naming Alcohol Groups Step 1: Find the alcohol (-OH) Step 2: Name the parent chain Step 3: Determine the carbon that the alcohol is coming off of Step 4: Drop the –e off the parent chain’s name Step 5: Add –ol to the end of the name Step 6: The number can be placed before name or between the parent chain and –ol Variations to Alcohol Groups Can have multiple alcohol groups off of one parent chain Named –diol, -triol, -tetraol, etc. Don’t take off the last e on the parent chain. Ethylene glycol 2,3-butandiol Variations cont… Can have parent chains other than alkanes Named as alkenol, alkynol, benzenol (phenol), etc. Can have iso-, sec-, tert- in naming Names the carbon that holds the alcohol t-butanol phenol Examples Practice Naming… Answers… ethanol pentan-3-ol Drawing… Step 1: draw parent chain; number of carbons will be indicated by prefix (i.e. meth, eth, prop…) Note bond type (i.e. -ene/-yne) Step 2: find the number carbon the group corresponds to Step 3: draw the attached group Examples… 1-propanol 2-methylhex-4-en-1,3-diol Answers… 1-propanol 2-methylhex-4-en-1,3-diol Priority…we’re #1 Greatest in priority… Alcohols Alkenes Alkynes Halides Nitro Alkanes phenyl Examples… Answers… 3-methylpentan-2-ol 3,7-dimethyl-6-octen-1-ol