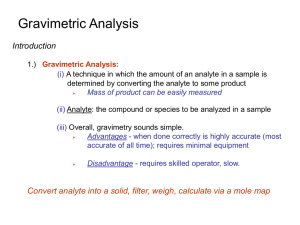
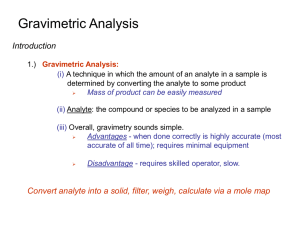
gravmetric analysis
advertisement
Gravimetric Analysis gravi – metric (weighing - measure) Gravimetric Analysis Gravimetric Analysis: is analytical technique based on the measurement of mass Steps of Gravimetric Analysis 1) Formation of precipitation 2) Isolation of precipitation 3) Determination of precipitation We are interested in knowing the purity of sample of NaCL obtained from seawater to do so we need To determine expermintally the precent by mass of CL in NaCL A + B → C↓ + D precipitation AgNO3(aq) + NaCl(aq) → AgCl (s)↓ + NaNO3(𝑎𝑞 ) Precipitation: first we would accuratly weigh out a sample NaCL and dissolve in water (0.5662g) Add enough ( AgNO3 aq) to the NaCL aq to cause the precipitation of all the CL- ions present in solution in solution (AgCL ) Filter precipitation Dry precipitation Weigh precipitation (1.0882) dissolved components sample precipitating agent 0.5662-g sample of an ionic compound containg chloride ions and an unknown metal is dissolved in water and treated with an excess of AgNO3. IF 1.0882 g of AgCL precipitate forms what is percent by mass of CL in original compound % CL = % CL = 𝐴𝑇𝑂𝑀𝐼𝐶 𝑀𝐴𝑆𝑆 𝑂𝐹 𝐶𝐿 𝑀𝑂𝐿𝐸𝐶𝑈𝐿𝐴𝑅 𝑀𝐴𝑆𝑆 𝑂𝐹 𝐴𝑔𝐶𝐿 35.45 143.4 X100 X1OO= 24.72% MASS OF CL = 0.42 X 1.0882 = 0.269 % CL = 0.27 0.566 X100 = 47.51%