Ch. 7 Alcohols and Phenols MAHWASH HAFEEZ BY
advertisement

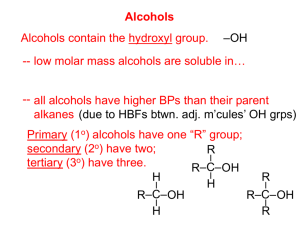
Ch. 7 Alcohols and Phenols BY MAHWASH HAFEEZ General Formulas and Functional Groups Both of these families contain a hydroxyl group (OH) as functional group Alcohols Phenols ALCOHOLS Bent molecule Central oxygen atom Hydrogen and alkyl group attached (R) to central “OXYGEN” atom (An alkyl group is formed by removing one hydrogen from any alkane) PHENOLS Ar – OH An aryl group attached to a hydroxyl “OH” group. (An aryl group is formed by removing one hydrogen from any a benzene ring) Classification and Nomenclature of Alcohols Alcohols can be of three main types depending upon the number of alkyl groups( carbon atoms) attached to the main carbon atom to which is the functional group attached. Primary (1°) carbon atom Secondary (2°) carbon atom Tertiary (3°) carbon atom Classification and Nomenclature of Alcohols Generally Primary Alcohol R – CH2 –OH Secondary Alcohol R2–CH – OH Tertiary Alcohol R3 –C – OH NOMENCLATURE (ALCOHOLS & PHENOLS) STEP 1 Select the longest chain containing the hydroxyl and replace the –e ending of the corresponding parent with –ol STEP 2 Number the carbons of the parent chain beginning at the end nearer the hydroxyl STEP 3 Number all substituents according to their position on the chain, and write the name listing the substituents in alphabetical order. STEP 4 If there are more than one substituents on the same chain then start the numbering from the side to give minimum sum of the positions of all those substituents. EXAMPLES Alkane Methane alcohol name Methanol Alcohol structure CH3-OH (methyl alcohol) Ethane Ethanol CH3-CH2-OH (ethyl alcohol) propane Propanol CH3-CH2-CH2-OH (propyl alcohol) propane 2-propanol (isopropylalcohol) butane Pentane hexane heptane 1-Butanol 1-Pentanol 1-Hexanol 1-Heptanol OH ǀ CH3-CH-CH3 CH3-CH2-CH2-CH2-OH CH3-CH2-CH2-CH2-CH2-OH CH3-CH2-CH2-CH2-CH2-CH2-OH CH3-CH2-CH2-CH2-CH2-CH2-CH2-OH PREPARATION OF ALCOHOLS 1. Hydration of Alkenes: Alcohols can easily be prepared on industrial scale by hydration of alkenes. CH2═CH2 + H2O CH3 ─ CH2─OH ethylene ethyl alcohol 2. Hydrolysis of alkyl Halides: Alcohols can be prepared by the hydrolysis of alkyl halides by means of water or an aqueous alkali. H O R – X+ NaOH 2 R – OH + NaX 3. Grignard synthesis: Any carbonyl compound when reacted with grignard reagent in the presence of water or a dilute acid gives an alcohol. O X RMgX + H –C – H H2O RCH2OH + Mg 4. Reduction of carbonyl compounds: OH Alcohols can be easily prepared by the reduction of aldehydes or ketones either by hydrogenation in the presence of metal catalysts such as nickel, palladium or platinum. O R – C - H RCH2OH O R – C – R R2CHOH ═ ═ ═ Physical Properties of Alcohols Alcohols are generally neutral colourless liquids but some alcohols particularly high molecular weights are solids. Not as homologous as alkanes They mostly have high melting and boiling points due to strong intermolecular attractions. The -OH groups of alcohols can hydrogen bond with one another and with other molecules. Solubility The more compact the molecule is,the more soluble it is 4-5 carbons or less—soluble in water. Markovnikov’s Rule The hydroxyl group goes on the carbon with fewer hydrogens Sec. 14.6 Chemical Properties of Alcohols Reactions Occur on the functional groups May involve hydrogen atoms as well Dehydration Oxidation Dehydration During dehydration of alcohols a water molecule is removed and ethylene is produced. Sulphuric acid is used as a catalyst. Oxidation ═ When alcohols are treated with oxygen, different alcohols yield different products. i.e. Primary alcohol yields an aldehyde on oxidation. O [O] RCH2OH R–C–H Secondary alcohols yields ketone on oxidation. O [O] R2CHOH R–C–R Tertiary alcohols generally do not react with oxygen. ═ PREPARATION OF PHENOLS 1. Fusion of sodium benzosulphonate with alkali: Benzene sulphonic acid fuses with sodium hydroxide to form sodium salt of benzene sulphonic acid which then produces phenol. 2. Hydrolysis of Chlorobenzene: In this process chlorobenzene is hydrolysed with 10% aqueous sodium hydroxide solution to produce phenol. heat 3. Oxidation of Cumene: Air oxidation of cumene results in the formation of phenol. cumene Physical properties of phenols They are sparingly soluble in water but solubility increases with increase in the number of hydroxyl ions. They are acidic in nature. Generally have high melting points They have high boiling points. Phenols Used as: Antiseptic Disinfectants First used was pure phenol—proved to be too toxic Methyl derivatives Cresols Creosote Phenols Dihydroxybenzenes Components of biochemical molecules Reactions of Phenols 1. Salt formation: Most phenols can be converted to their salts by aqueous sodium hydroxide. 2. Ester formation: Phenols can be allowed to form esters by allowing them to react with an acid chloride or anhydride in the presence of an acid or a base. aq.NaOH NaCl +H O 2 3. Williamson ether synthesis: Phenols form alkyl or aryl ethers on treatment with alkyl halides in the presence of aqueous sodium hydroxide. 4. Nitration: Phenol on treatment with conc. nitric acid is converted into 2,4,6- trinitrobenzene.