Electromagnetic Waves
advertisement

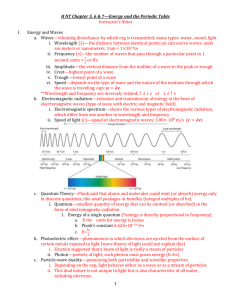
Chapter 11 Atomic Structure • If atoms are too small to see, obviously electrons are beyond our vision. • We get clues from the atom on where its electrons are located from energy it absorbs and releases. • This energy is in the form of waves of various types from x-rays to radio waves; all of which are classified as electromagnetic waves. Electromagnetic Waves • A wave transmits energy from the source to the receiver. • Electromagnetic waves do this by disturbing the electric and magnetic fields surrounding the earth. • The types of electromagnetic waves are arranged on a spectrum from greatest to smallest frequency. Electromagnetic Spectrum Color is Frequency Media Frequencies • AM radio - 535 kilohertz to 1.7 megahertz • Short wave radio - bands from 5.9 megahertz to 26.1 megahertz • Citizens band (CB) radio - 26.96 megahertz to 27.41 megahertz • Television stations - 54 to 88 megahertz for channels 2 through 6 • FM radio - 88 megahertz to 108 megahertz • Television stations - 174 to 220 megahertz for channels 7 through 13 Household Gadgets Frequencies • Garage door openers, alarm systems, etc. Around 40 megahertz • Standard cordless phones: Bands from 40 to 50 megahertz • Baby monitors: 49 megahertz • Radio controlled airplanes: Around 72 megahertz, which is different from... • Radio controlled cars: Around 75 megahertz • Wildlife tracking collars: 215 to 220 megahertz • Cell Phones: 824 to 849 megahertz • New cordless phones: around 900 megahertz! Electromagnetic Spectrum Wave Properties • Wavelength (λ) is the distance between two successive waves. • Frequency (f) is the number of waves passing a point per second. Wave Properties Wave Properties • v = f * λ wave speed equation • Wave speed (v) is the speed of electromagnetic waves. In a vacuum this is 3.0 x 108 m/s or 186,000 miles per second! • This is referred to as the “speed of light” though all radiation travels at this speed. Energy of E/M Waves • The energy of E/M radiation is proportional to frequency. • The more energetic waves are higher in frequency like x-rays. • E = h* f • h is a constant in nature • h = 6.626 x 10-34 J/Hz Example • What is the frequency and energy of green light which has a wavelength of 510 nm? • f = c/ λ = 3.0e8/510e-9 = 5.9e14 hz • E = hf = 6.6e-34 * 5.9e14 = 3.9e-19 J Bohr Model of the Atom • To explain the emission of light, Bohr came up with a radical model of the atom which had electrons orbiting around a nucleus. • This similarity between a planetary model and the Bohr Model of the atom ultimately arises because the attractive gravitational force in a solar system and the attractive Coulomb (electrical) force between the positively charged nucleus and the negatively charged electrons in an atom are mathematically of the same form. Energy Levels of the Atom • In the Bohr model, electrons can jump to higher energy states by the addition of energy of certain frequencies • The excited electron is unstable and falls down to ground state giving off electromagnetic energy equal in frequency to the energy change. Hydrogen Emission Series • The electrons can move to only certain locations from the nucleus • The result of this restriction in movement is specific and distinct wavelengths of EM radiation is given off as the electrons transition. Energy Quantified • The energy transitions are quantified and thus the transitions accept and release only certain energies of E/M radiation. • The energy of each level for the hydrogen atom is given by the equation • En = RH/n2 • RH is called the Rydberg constant • RH = 2.180 x 10-18 J Atomic Spectra • White light emits all colors because a multitude of electron transitions occur between energy levels. • Spectral Lines give evidence of electron transitions. • A specific species of gas emits only a limited amount of spectral colors because of a more limited availability of electron transitions. Emission Spectra for H2 and He More Emission Spectra Na, Ne, Hg