CHEM 1411 Chapter 7.doc
advertisement

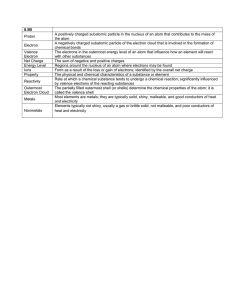
CHEM 1411- CHAPTER 7 ATOMIC STRUCTURE & PERIODICITY Electro-Magnetic Radiation Radiations like visible light, X-rays, UV radiations, IR radiations, cosmic rays etc are called electromagnetic radiations. They are associated with electric and magnetic fields. They are produced by the oscillation or vibration of charged particles. . All electromagnetic radiations travel with the same velocity. They are charge less and do not contain any material particles. Nature of Electro Magnetic radiations 1.Wave nature According to Clerk Maxwell , Electromagnetic radiations travel continuously in the form of waves. Each radiation has a definite wavelength and all other properties associated with the waves. C = Where, ‘C’ is the velocity of radiation, ‘’ is the frequency and ‘ ‘ is the wavelength 2.Particle nature. (Quantum Theory) According to Max Planck, electromagnetic radiations travel discontinuously in the form of minute packets of energy. The energy of each packet is given as E=hv ‘ h ‘ is Planck’s constant (6.63 x 10 –34 J.s) and ‘v’ is the frequency of radiation These energy packets are called ‘quanta’ and the energy of each packet is called a quantum of energy. In visible light, these energy packets are known as photons. Photoelectric Effect The phenomenon of the ejection of electrons from the surface of metals when they are exposed to radiations of certain frequency. The minimum frequency needed for the ejection of electrons is called the ‘Threshold Frequency’ Spectrum A Spectrum is an arrangement of electromagnetic radiations in the increasing order of their wavelengths. Light from the sun, a lit candle or an incandescent bulb is called white light. This when resolved through a prism, gives a continuous spectrum. Here the colors (wavelengths) are arranged in the order VIBGYOR. Atomic Spectra When energy is given to an atom in the form of heat energy or electrical energy, the electrons in the atom get excited to higher energy levels by absorbing energy. This is the excited state of an atom, which is unstable. The electrons then start falling from higher levels to lower levels, releasing energy. This energy when resolved through a spectroscope, we get different lines of specific wavelengths. This is called Atomic Emission Spectrum or the Line Spectrum. This is a discontinuous spectrum. When radiations pass through some substances, certain wavelengths are absorbed, giving an Absorption Spectrum. Each element has its own Absorption spectrum. So, it is used as a tool for qualitative and quantitative chemical analysis. Bohr’s Theory of the Hydrogen atom The hydrogen atom has only one electron, which is present in the first shell. When energy is supplied to the ‘H’ atoms the electrons get excited to higher energy levels by absorbing energy. This state is unstable and the electrons start falling from higher levels to lower levels by releasing energy. This emitted energy appears as discrete lines in the spectrum of Hydrogen. The energy released is given by E=Ef–Ei 1 E = hv = RH 1 - ni2 nf2 Where, RH is Rydberg’s constant for ‘H’ atom,(2.18 x 10-18 J) , n i = initial state, nf = final state The different series of lines in H spectrum are Lyman (UV), Balmer (V), Paschen (IR), Brackett (IR) and Pfund (IR). Dual Nature of Electron Louis de Broglie suggested that like the electromagnetic radiations, the electrons and other material particles also possess a dual nature i.e. they behave as a particle as well as a wave. de Broglie’s equation = h / mu Where, ‘’ is the wavelength, ‘h’ is Planck’s constant and ‘u’ is the velocity of electron. Heisenberg’s Uncertainty principle The principle states that it is impossible to find simultaneously both position and momentum of a small moving particle like an electron. This is mathematically expressed as x x p = h / 4 x = Uncertainty in finding the position p = Uncertainty in finding the Momentum Orbital Heisenberg’s Uncertainty principle and de Broglie’s concept lead to the idea that the electrons in the atom are present in different orbital. An orbital is a region of space around the nucleus where there is maximum possibility of finding the electron. Thus the electrons in the atom are distributed in different Shells, Sub shells and Orbital. Quantum numbers These numbers help to locate the position of electrons in the atom 1.Principal quantum Number (n) This the most important quantum number because most of the energy of the electrons is associated with this quantum number. It represents the main energy level or Shell n = 1,2,3,4, …. 2. Angular momentum quantum number ( l ) This quantum number represents the sub shells in the atom. The sub shells are designated as s, p , d , f, g and h The values of ‘ l ‘ are l = 0 to (n –1) 3. Magnetic Quantum Number (m) This quantum number represents the orbital in the atom. The values of ‘ m ‘ are m = - l, 0, +l 4. Spin quantum number (s) This quantum number arises due to the spinning of electrons about its own axis. The spinning can be either in clockwise or anticlockwise direction. The values of ‘s’ are + ½ and - ½ m # of orbital max# of e- s 0 1 2 0 s 0 1 2 1 p -1,0,+1 3 6 0 s 0 1 2 1 p -1,0,+1 3 6 2 d -2,-1,0,+1,+2 5 10 0 s 0 1 2 1 p -1,0,+1 3 6 2 d -2,-1,0,+1,+2 5 10 3 f -3,-2,-1,0,+1,+2,+3 7 14 n l 1 0 2 3 4 sub shell Electron configuration of atoms The following rules govern the electron configuration in atoms 1.The Pauli exclusion principle States that no two electrons in an atom can have the entire four quantum numbers same. The principle follows that an orbital cannot accommodate more than two electrons. 2.Hund’s rule The rule states that pairing of electrons any orbital of the atom is not possible until all the available orbital of the given set contain one electron each. 3.Aufbau principle ( Aufbau is a German word meaning “ build up “ The principle states that electrons are filled in different orbital of the atom in the order of increasing energy. Principal quantum # n =1 1s 2 2s 2p 3 3s 3p 3d 4 4s 4p 4d 4f 5 5s 5p 5d 5f 6 6s 6p 6d 7 7s 7p Periodicity in Properties of Elements 1. Effective nuclear Charge The shielding effect of the inner electrons and increase in the size of the atom decreases the electrostatic forces of attraction between the nucleus and the valence electrons. The shielding effect of electrons decreases in the order s > p > d > f.This will reduce the effective nuclear charge. Effective nuclear Charge of elements increases across a period and decreases down the group. 2. Atomic radius It is half of the distance between the two nuclei of two adjacent atoms in a metal or a diatomic molecule. Atomic radius decreases across a period because of the increase in effective nuclear charge on the valence electrons. But atomic radius increases down the group as the effective nuclear charge on the valence electrons decreases due to the addition of more shells. 3. Ionic radius Radius of a cation is always smaller than that of the corresponding atom because a cation is formed by the loss of one or more electrons and this causes an increase in effective nuclear charge on the valence electrons. On the other hand the radius of an anion is always larger than that of the corresponding atom because an anion is formed by the gain of one or more electrons and this causes a decrease in effective nuclear charge on the valence electrons. 4. Ionization energy (KJ / mol) The minimum amount of energy required to remove an electron from the valence shell of a neutral gaseous atom in the ground state is called the first ionization energy (I1) The minimum amount of energy required to remove an electron from the valence shell of a mono positive gaseous ion in the ground state is called the second ionization energy (I2) I1 < I2 < I3 <…. In most cases the ionization energy of elements increases from left to right in the period because, Increase in effective nuclear charge Decrease in atomic radius Both conditions require more energy to remove the electron from the valence shell Ionization energy of elements decreases down the group because, Decrease in effective nuclear charge Increase in atomic radius 5. Electron Affinity The energy released when an electron is added to the valence shell of a neutral gaseous atom in its ground state is called the electron affinity. Electron affinity of elements increases from left to right in the period because of the increase in effective nuclear charge on the valence electrons. Electron affinity of elements decreases down the group in the periodic table because of the decrease in effective nuclear charge on the valence electrons. Electron affinity of Fluorine is lesser than that of Chlorine because of its small size.