Acetate Buffer Lab.doc
advertisement

Acetate Buffer
Introduction
Buffers are solutions that resist a change in pH when small amounts of acid or base are
added. They contain an acidic component to neutralize OH- ions and a basic component
to neutralize H+ ions. Thus, it is essential that these two components must be able to
coexist in a solution without completely neutralizing each other. Often buffers are made
of weak acid-base conjugate pairs, for example, acetic acid, HC2H3O2 with its base
C2H3O2-.
If we have a weak acid, HX, and its conjugate base, X-, the following equilibrium occurs:
HX(aq) ↔ H+(aq) + X-(aq)
where X- is the conjugate base.
Ka = { [H+][X-] / [HX] }
If an amount of H+ ions are added, the decrease in pH is counteracted by the equilibrium
reaction shifting to the left. This will cause the [H+] to decrease, staying close to what it
was originally and holding the pH fairly constant.
If OH- ions are added they will remove H+ ions to form water. The increase of pH is
counteracted by the equilibrium reaction shifting to the right as H+ ions are removed.
The [H+] will remain close to what it was originally, holding the pH fairly constant.
The most useful buffering solutions are those which have similar concentrations of HX
and X-, giving the buffer the capacity to absorb acid or base with the same effectiveness.
When choosing an appropriate conjugate acid-base pair to form a buffer at a specific
pH, the most effective buffers have a required pH within 1.0 of the conjugate acid's
pKa, where pKa =-log Ka.
The buffering capacity is the amount of acid or base a buffer can accept without the pH
changing appreciably. The greater the amounts of the conjugate acid-base pair, the more
resistant they are to change in pH.
If we assume that the amount of added acid or base is less than 5% of the conjugate
acid/base molarity and solve the acid-dissociation-constant expression for [H+] we get:
[H+] = Ka { [HX] / [X-] }
We can use this to determine the pH of a buffer:
We first take the negative log of both sides:
-log [H+] = -log [Ka { [HX] / [X-] }] = -log Ka - log { [HX] / [X-] }
We know -log [H+] = pH and -log Ka = pKa.
pH = pKa - log { [HX] / [X-] } = pKa + log { [X-] / [HX] }
In general:
pH = pKa + log { [base] / [acid] }
This is known as the Henderson-Hasselbalch equation.
Acetate Buffer
Procedure
**NOTE: Use instructions provide by the instructor – DO NOT USE INSTRUCTIONS
IN CHEMLAB! To remove the instructions on the screen, and free-up more working
area, perform the following operation: click on the OPTIONS tab; then click on LAB
ONLY. The instructions “disappear” and all of the area is now lab space.**
Preparation of the buffer solution:
1.
2.
3.
4.
5.
6.
7.
8.
Obtain a 600 mL Beaker from the Equipment menu.
Select the Beaker and add a pH meter (from the Equipment menu).
Add 74 mL of 0.1 M Acetic Acid C2H4O2.
Add 1.1 g of Sodium Acetate NaC2H3O2
Add 26 mL of Water (from chemicals dialog box).
Stir until completely dissolved.
Record the initial pH of the buffer solution.
Make a copy of buffer solution and set Beaker aside.
Titration of buffer with a strong base:
1. Select the 600 mL Beaker containing the buffer solution and turn on the collection of
titration data by selecting the Collect Titration Data from the Procedures menu (or
from the right-mouse context menu). Since only one set of data can be collected per
lab, you will be asked to drop the previously collected data.
2. Add a 50 mL burette to the lab.
3. Fill the burette with 50 mL of 0.1 M NaOH solution
4. Place Erlenmeyer flask solution on stir plate, Select Equipment→ Hot plate and mag
stir – place the Erlenmeyer flask on the plate and turn on the magnetic stir bar.
5. Open the Titration data window by selecting View Titration Data from the Procedures
menu.
6. Add 100 mL of NaOH (when first 50 mL have been added, close petcock and refill
the burette. Continue recording titration data)
7. Add a title and labels to the generated curve.
8. Copy the curve from the Titration data window to Word. Print and include with your
report.
Titration of buffer with a strong acid:
1. Select the 600 mL Beaker containing the buffer solution and turn on the collection of
titration data by selecting the Collect Titration Data from the Procedures menu (or
from the right-mouse context menu). Since only one set of data can be collected per
lab, you will be asked to drop the previously collected data.
2. Add a 50 mL burette to the lab.
3. Fill the burette with 50 mL of 0.1 M HCl solution
4. Place Erlenmeyer flask solution on stir plate, Select Equipment→ Hot plate and mag
stir – place the Erlenmeyer flask on the plate and turn on the magnetic stir bar.
5. Open the Titration data window by selecting View Titration Data from the Procedures
menu.
6. Add 200 mL of HCl (when first 50 mL have been added, close petcock and refill the
burette and continue recording titration data until 200 mL have been delivered)
7. Add a title and labels to the generated curve.
8. Copy the curve from the Titration data window to Word. Print curve.
Acetate Buffer
Observations
Name:__________
Sect:____________
Data
Iinitial pH of buffer solution:
pH: __________
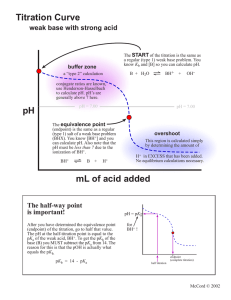
From the equivalence point, (mid point of the steepest part of titration curve), determine
the following:
Titration of buffer with a strong base:
pH of buffer solution (equivalence point):
Amount of NaOH added (equivalence point):
pH: __________
__________mL
Titration of the buffer with a strong acid:
pH of buffer solution (equivalence point):
Amount of HCl added (equivalence point):
pH: __________
__________mL
Calculations
Calculating the expected pH of the buffer solution:
1. Given that the pKa for Acetic Acid is 4.77, calculate the expected pH of the buffer
solutions using the Henderson-Hasselbalch equation and the concentrations of Acetic
Acid and Acetate added to the 250 ml Erlenmeyer flask:
pH = pKa + log { [base] / [acid] }
=?
Hints:
moles of base = mass of NaC2H3O2 / MW of NaC2H3O2
[base] = moles of base / total initial volume
moles of acid = volume of C2H4O2 in liters x molarity of C2H4O2
[acid] = moles of acid / total initial volume
pH = _______
2. Calculate the expected pH of the following solutions - use the logic in calculation #1
for guidance:
2-A
50.0 mL 0.10 M NH3 + 50 mL 0.10 M NH4NO3 (pKa = 9.25)
pH = _______
2-B
10 mL of solution 2-A + 5 mL H2O + 1mL 0.10 M HCl (pKa = 9.25)
pH = _______
2-C
10 mL of solution 2-A + 6 mL 0.10 mL HCl (pKa = 9.25)
pH = _______
2-D
10 mL of solution 2-A + 5 mL H2O + 1 mL 0.10 M NaOH (pKa = 9.25)
pH = _______
3. Should the pH of a buffer change when the buffer is diluted? Explain fully, using the
Henderson-Hasselbalch equation:
pH = pKa + log { [base] / [acid] }
Questions
1. What conclusions can you draw from looking at the titration curve of the weak acid
(Weak Acid Titration Lab) with a strong base and comparing it to the titration curve for
the buffer with a strong base?
2. What conclusions can you draw from looking at the titration curve of the buffer with a
strong base and comparing it to the titration curve for the buffer with a strong acid?
Discussion