1411 Chapter 8 Practice Problems.doc
advertisement

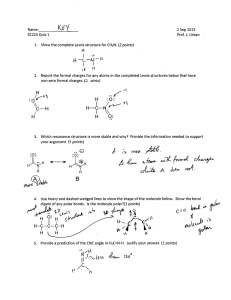
CHEM 1411 – Chapter 8 Chemical bonding - I: Basic Concepts 1. Write electron configuration, Lewis dot symbols for Nitrogen and Silicon and determine total valence electrons. 3. Draw the Lewis symbols (electron dot symbols) for each of the following atoms or ions: Cl Al3+ O2Ar N34. Write the chemical formula for the ionic compound formed between following pairs of elements: a) Ca and F b) Mg and N c) Lithium and O d ) Barium and Iodine e) Cr3+ and O2- 5. Determine the one with larger lattice energy (circle one) in each of the following pairs. (Please give a reason) a) CaO, MgO b) NaF, MgO c) MgO, MgS d) Fe2O3 , FeO 7. Using only periodic table, select the most electronegative atom in each of the following sets: a) As, Si, Ge, Ga b) Li, Be, B, Al c) Cl, F, I, Br 8. Using electronegativity table classify the following as ionic, polar covalent, or non-polar covalent bonds; a) H-Br b) K-H c) Na-I d) Br-Br e) N-H 9. Which of the following bonds are polar? (circle the more polar or more electronegative one) a) B-Cl b) P-F c) Br-Cl d) O-Br e) Hg-Sb f) N-H, C-H, O-H g) I-F, Br-Cl, I-Br 10. Determine the formal charge of each atom in each of the followings; .. .. a) :N = C= S: b) :C == N: c) S = Si Cl H 14. Using the bond enthalpies table (bond dissociation energies in KJ/mol), estimate enthalpy change for each of the following gas-phase reactions: H H H H a) H - C ---- C - O - H (g) H - C –O- C - H (g) H H H H H H b) H - C - N Ξ C H - C - C Ξ N H H c) C2H4 (g) + 3 O2 (g) 2 CO2 (g) + 2 H2O (g)