Zumdahl - 1411 Chapter 9 Practice Problems.doc
advertisement
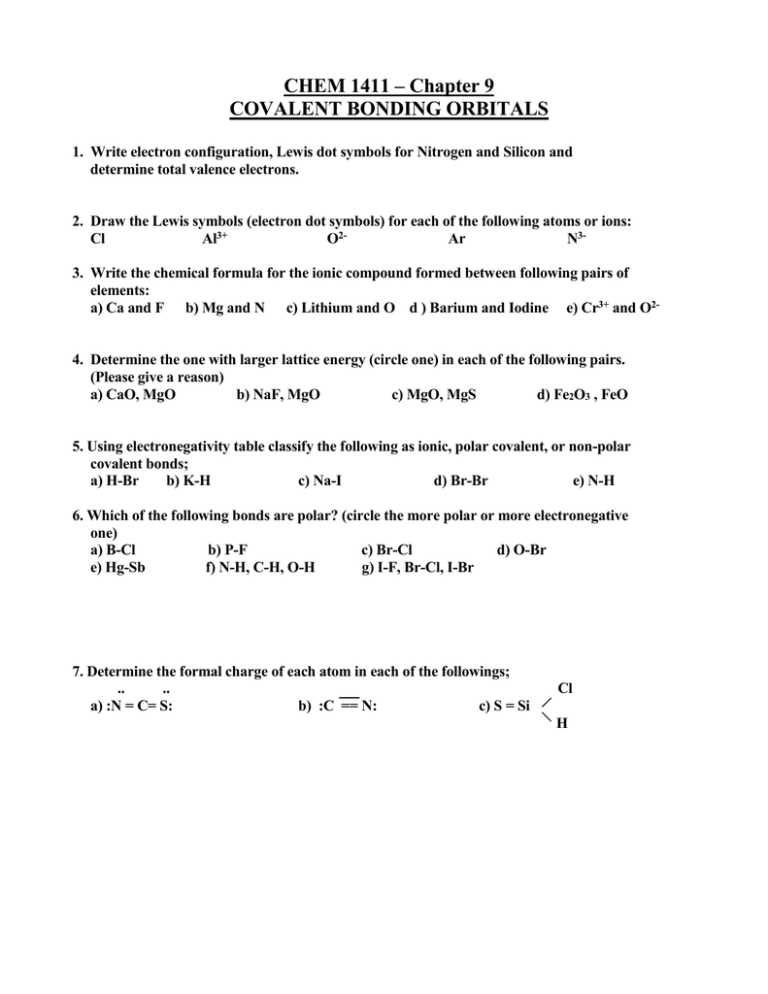
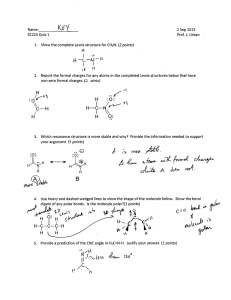
CHEM 1411 – Chapter 9 COVALENT BONDING ORBITALS 1. Write electron configuration, Lewis dot symbols for Nitrogen and Silicon and determine total valence electrons. 2. Draw the Lewis symbols (electron dot symbols) for each of the following atoms or ions: Cl Al3+ O2Ar N33. Write the chemical formula for the ionic compound formed between following pairs of elements: a) Ca and F b) Mg and N c) Lithium and O d ) Barium and Iodine e) Cr3+ and O2- 4. Determine the one with larger lattice energy (circle one) in each of the following pairs. (Please give a reason) a) CaO, MgO b) NaF, MgO c) MgO, MgS d) Fe2O3 , FeO 5. Using electronegativity table classify the following as ionic, polar covalent, or non-polar covalent bonds; a) H-Br b) K-H c) Na-I d) Br-Br e) N-H 6. Which of the following bonds are polar? (circle the more polar or more electronegative one) a) B-Cl b) P-F c) Br-Cl d) O-Br e) Hg-Sb f) N-H, C-H, O-H g) I-F, Br-Cl, I-Br 7. Determine the formal charge of each atom in each of the followings; .. .. a) :N = C= S: b) :C == N: c) S = Si Cl H 8. Using the bond enthalpies table (bond dissociation energies in KJ/mol), estimate enthalpy change for each of the following gas-phase reactions: H H H H a) H - C ---- C - O - H (g) H - C –O- C - H (g) H H H H H H b) H - C - N Ξ C H - C - C Ξ N H H c) C2H4 (g) + 3 O2 (g) 2 CO2 (g) + 2 H2O (g) 9. Using VSEPR model, predict the molecular geometry (molecular shape) and electron geometry of the followings: O3 CO32H2O NF3 10. Indicate the hybridization of orbitals by the central atom in each of the following; SO32SO3 NH4+ SCl2 11. Predict the approximate value for the bond angles for each carbon indicated in the following compounds: H H (a) H - C - C C -H H (b) H - C - O -H H 12. Draw Lewis dot structures for following molecules and ions, predict which will exhibits delocalized pi () bonding ( resonance structure). SO32NH4+ CHCl3 I313. Identify the following molecules or ions as stable or unstable. Explain why? Compare the stability. He2 He2+ O2 O2N2