Eu 3+ Doped Y 2 O 3 Nanocrystals
advertisement
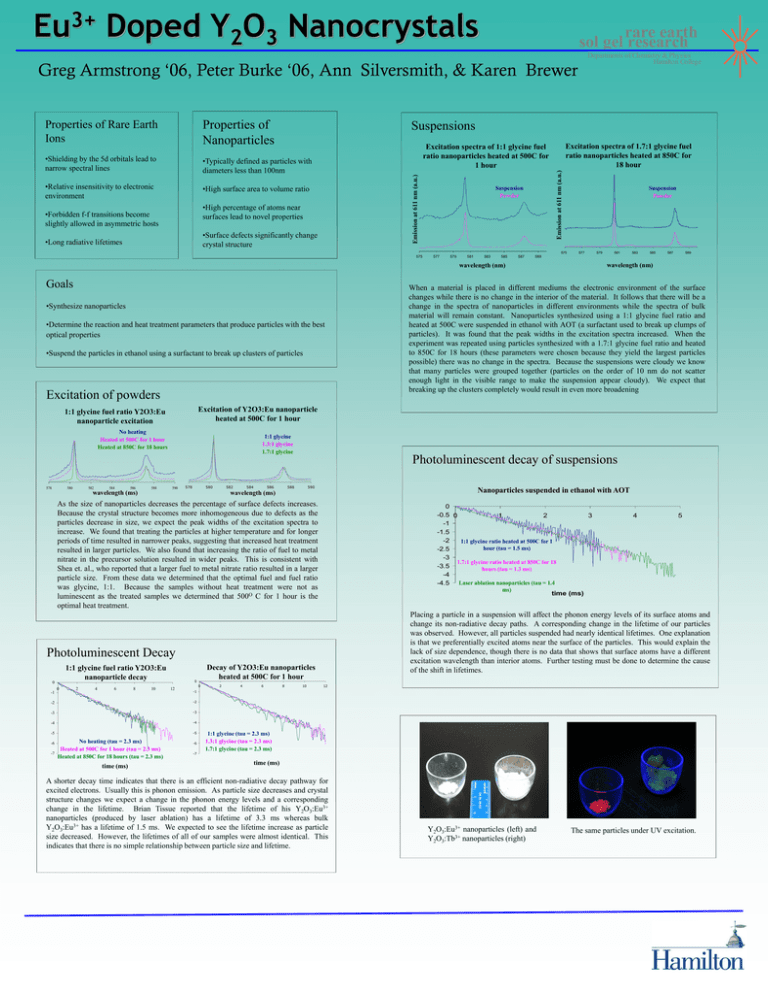
3+ Eu Doped Y2O3 Nanocrystals rare earth sol gel research Departments of Chemistry & Physics Hamilton College Greg Armstrong ‘06, Peter Burke ‘06, Ann Silversmith, & Karen Brewer Properties of Rare Earth Ions Properties of Nanoparticles •Shielding by the 5d orbitals lead to narrow spectral lines •Typically defined as particles with diameters less than 100nm •Relative insensitivity to electronic environment •High surface area to volume ratio Suspensions •Surface defects significantly change crystal structure •Long radiative lifetimes Emission at 611 nm (a.u.) Emission at 611 nm (a.u.) •High percentage of atoms near surfaces lead to novel properties •Forbidden f-f transitions become slightly allowed in asymmetric hosts Excitation spectra of 1.7:1 glycine fuel ratio nanoparticles heated at 850C for 18 hour Excitation spectra of 1:1 glycine fuel ratio nanoparticles heated at 500C for 1 hour Suspension Powder 575 575 577 579 581 583 585 587 Suspension Powder 577 579 •Synthesize nanoparticles •Determine the reaction and heat treatment parameters that produce particles with the best optical properties •Suspend the particles in ethanol using a surfactant to break up clusters of particles Excitation of powders No heating Heated at 500C for 1 hour Heated at 850C for 18 hours 580 582 584 586 wavelength (ms) 588 1:1 glycine 1.3:1 glycine 1.7:1 glycine 590 578 580 582 584 586 588 wavelength (ms) 590 1:1 glycine fuel ratio Y2O3:Eu nanoparticle decay -1 2 4 6 8 10 12 0 ms) -3 -3 -4 -4 -5 -5 -7 time (ms) 2 4 6 8 10 3 4 5 time (ms) Placing a particle in a suspension will affect the phonon energy levels of its surface atoms and change its non-radiative decay paths. A corresponding change in the lifetime of our particles was observed. However, all particles suspended had nearly identical lifetimes. One explanation is that we preferentially excited atoms near the surface of the particles. This would explain the lack of size dependence, though there is no data that shows that surface atoms have a different excitation wavelength than interior atoms. Further testing must be done to determine the cause of the shift in lifetimes. 12 -1 -2 No heating (tau = 2.3 ms) Heated at 500C for 1 hour (tau = 2.3 ms) Heated at 850C for 18 hours (tau = 2.3 ms) When a material is placed in different mediums the electronic environment of the surface changes while there is no change in the interior of the material. It follows that there will be a change in the spectra of nanoparticles in different environments while the spectra of bulk material will remain constant. Nanoparticles synthesized using a 1:1 glycine fuel ratio and heated at 500C were suspended in ethanol with AOT (a surfactant used to break up clumps of particles). It was found that the peak widths in the excitation spectra increased. When the experiment was repeated using particles synthesized with a 1.7:1 glycine fuel ratio and heated to 850C for 18 hours (these parameters were chosen because they yield the largest particles possible) there was no change in the spectra. Because the suspensions were cloudy we know that many particles were grouped together (particles on the order of 10 nm do not scatter enough light in the visible range to make the suspension appear cloudy). We expect that breaking up the clusters completely would result in even more broadening 0 -0.5 0 1 2 -1 -1.5 -2 1:1 glycine ratio heated at 500C for 1 hour (tau = 1.5 ms) -2.5 -3 1.7:1 glycine ratio heated at 850C for 18 -3.5 hours (tau = 1.3 ms) -4 Laser ablation nanoparticles (tau = 1.4 -4.5 Decay of Y2O3:Eu nanoparticles heated at 500C for 1 hour 0 -2 -6 589 Nanoparticles suspended in ethanol with AOT Photoluminescent Decay 0 587 Photoluminescent decay of suspensions As the size of nanoparticles decreases the percentage of surface defects increases. Because the crystal structure becomes more inhomogeneous due to defects as the particles decrease in size, we expect the peak widths of the excitation spectra to increase. We found that treating the particles at higher temperature and for longer periods of time resulted in narrower peaks, suggesting that increased heat treatment resulted in larger particles. We also found that increasing the ratio of fuel to metal nitrate in the precursor solution resulted in wider peaks. This is consistent with Shea et. al., who reported that a larger fuel to metal nitrate ratio resulted in a larger particle size. From these data we determined that the optimal fuel and fuel ratio was glycine, 1:1. Because the samples without heat treatment were not as luminescent as the treated samples we determined that 500O C for 1 hour is the optimal heat treatment. 0 585 Excitation of Y2O3:Eu nanoparticle heated at 500C for 1 hour 1:1 glycine fuel ratio Y2O3:Eu nanoparticle excitation 578 583 wavelength (nm) wavelength (nm) Goals 581 589 -6 -7 1:1 glycine (tau = 2.3 ms) 1.3:1 glycine (tau = 2.3 ms) 1.7:1 glycine (tau = 2.3 ms) time (ms) A shorter decay time indicates that there is an efficient non-radiative decay pathway for excited electrons. Usually this is phonon emission. As particle size decreases and crystal structure changes we expect a change in the phonon energy levels and a corresponding change in the lifetime. Brian Tissue reported that the lifetime of his Y2O3:Eu3+ nanoparticles (produced by laser ablation) has a lifetime of 3.3 ms whereas bulk Y2O3:Eu3+ has a lifetime of 1.5 ms. We expected to see the lifetime increase as particle size decreased. However, the lifetimes of all of our samples were almost identical. This indicates that there is no simple relationship between particle size and lifetime. Y2O3:Eu3+ nanoparticles (left) and Y2O3:Tb3+ nanoparticles (right) The same particles under UV excitation.






