lecture 11 - refrigerators and Carnot
advertisement

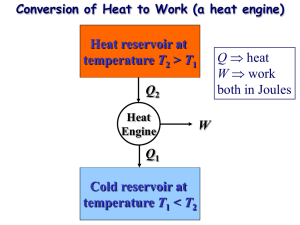
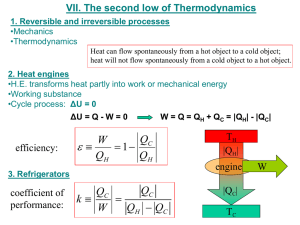
Announcements 9/24/10 8 days left to get your clicker registered I emailed two comments about HW 10-6 Vote on times for exam review session by tomorrow Happy Birthday, Dr. Colton Flagstaff Review Engines a. Picture b. Relationship between Qh, Qc, and |W| c. Defn of efficiency d. How to calculate efficiency Reading Quiz What is the “Clausius statement” of the Second Law of Thermodynamics? a. Adiabatic processes are reversible. b. Heat energy does not spontaneously flow from cold to hot. c. It is impossible to convert any heat into work. d. No real engine can be more efficient than the equivalent “Carnot engine”. e. There are no truly “irreversible” processes. Refrigerators (or air conditioners) heat, Qc fridge exhaust, Qh work COP: How good is your refrigerator? Heat Pumps heat, Qc heat pump “exhaust”, Qh work COP: How good is your heat pump? “Reversible” vs. “Irreversible” “In order for a process to be [totally*] reversible, we must return the gas to its original state without changing the surroundings.” Thought question: Is this [totally] reversible? a. Yes P state B; TB = 650K b. No c. Maybe state A; T = 300K A V *Other books’ terminology: reversible vs totally reversible. Carnot Cycle All heat added/subtracted reversibly a. During constant temperature processes b. Drawback: isothermal = slow, typically Tc " eC " 1 Th “C” for “Carnot” HW 11-5 – 11-7: find efficiency for a specific Carnot cycle Optional HW: eC derived for a general Carnot cycle Carnot Theorem Second Law, Kelvin-Plank statement a. You can’t fully convert heat to work b. You can’t have an efficiency of 100% Carnot Theorem: a. You can’t even have that! emax Tc eC 1 Th Th = max temp of cycle Tc = min temp of cycle Carnot Theorem: How to remember Engine: emax = ? Refrigerator: COPmax = ? Heat pump: COPmax = ? Carnot Theorem: Proof Part 1 of proof: The Kelvin-Plank statement of the Second Law is equivalent to the Clausius statement. Clausius: Heat energy does not spontaneously flow from cold to hot. Kelvin-Plank: You can’t fully convert all heat to work. What if you could make heat go from coldhot? Then do this: work heat engine exhaust What if you could make a perfect engine? Then use it to power a refrigerator. Carnot Theorem: Proof Part 2 of proof: A totally reversible engine can be run backwards as a refrigerator. (Obvious? It’s really: “Only a totally reversible…”) Why not this? Bottom line: you could build a system to do that, but it couldn’t be built from an engine/heat reservoirs that look like this: P P V V Carnot Theorem: Proof Part 3 of proof: Suppose you had an engine with e > emax. Then build a Carnot engine using the same reservoirs, running in reverse (as a fridge). Use the fridge’s heat output to power the engine: work Qc work fridge Qh engine exhaust (at Tc) Which work is bigger? Can you see the problem? Multi-Stage Carnot Engine? Build a new cycle using only isotherms and adiabats. Result? “Regeneration” …so you know something Dr. Durfee doesn’t …and so you engineers know a little about what’s coming The other way that you can transfer heat without changing entropy: internal heat transfer The Brayton cycle: Used by most non-steam power plants Isothermal contour Brayton cycle, cont. What does temperature look like at each point? Use “T-S” diagram. “S” = entropy, we’ll talk much more about on Monday For now, just know that adiabatic = constant S. Focus on y-axis Look here! Brayton cycle with regeneration Add another compressor & another turbine to increase the range over which regeneration can be done With an infinite number of compressors/turbines, you get the Carnot efficiency! (even with const. pressure sections) Image from http://web.me.unr.edu/me372/Spring2001/The%20Brayton%20Cycle%20with%20Regeneration.pdf (who apparently got it from a textbook)