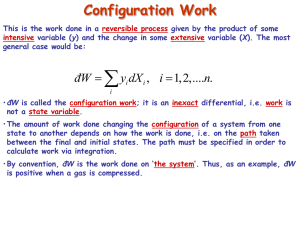
Heat reservoir at T Cold reservoir at Q
advertisement

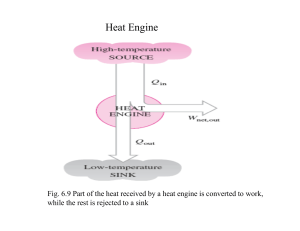
Conversion of Heat to Work (a heat engine) Heat reservoir at temperature T2 > T1 Q heat W work both in Joules Q2 Heat Engine Q1 Cold reservoir at temperature T1 < T2 W Cooling via Work (Carnot Refrigerator) Environment at temperature Th > Tl Qh W Refrigerator Ql Ql R Ql / W Qh Ql Tl Th Tl Refrigerator, inside temperature Tl < Th Carnot Heat Pump Environment (Home) Th > Tl Qh W Heat Pump Ql HP Qh Qh / W Qh Ql Th Tl 1 1 Th Tl Th Tl Resevoir (ground) Tl < Th Always > 100% The Clausius Inequality and the 2nd Law P Divide any reversible cycle into a series of thin Carnot cycles, where the isotherms are infinitesimally short: đQ2 đQ1 v • We have proven that the quantity dS = dQr/T is a state variable, since its integral around a closed loop is equal to zero, i.e. the integration of differential entropy, dS, is path independent! The Clausius Inequality and the 2nd Law P Divide any reversible cycle into a series of thin Carnot cycles, where the isotherms are infinitesimally short: đQ2 đQ1 v For a reversible process! Leads to the definition of entropy for a reversible process: đQr dS = T The Clausius Inequality and the 2nd Law P Divide any reversible cycle into a series of thin Carnot cycles, where the isotherms are infinitesimally short: đQ2 đQ1 v • There is one major caveat: the cycle must be reversible. In other words, the above assumes only configuration work (PdV) is performed. • If the cycle additionally includes dissipative work, it is not clear how to include this in the above diagram.