lecture 11 - refrigerators and Carnot
advertisement

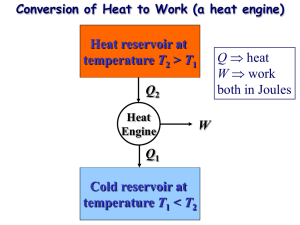
Announcements – 9/21/12 Prayer Remote Desktop check Exam starts a week from tomorrow a. Avoid Oct 1 and Oct 3 if possible Results of doodle.com voting: a. Exam review will be Wed 5-6:30, room to be announced Pearls Before Swine From warmup Extra time on? a. Example of a near-perfect Carnot engine; especially if a video of one exists (sped up, of course), or if we could see a demo of a Carnot engine in class. Other comments? a. I really don't get irreversibility. Can't you adiabatically compress and then immediately decompress a gas? If it's insulated well enough, don't you get nearly all the energy back? Worked Problem from last time P 303000 Pa 202000 Pa 3, 320 K Qh Qh 2 Qc 1, 320K V V2=? T2=? 0.001 m3 Game plan: a.Find unknown state variables b.Find Q for each leg c.Find |Wnet| d.Then e = |Wnet|/Qh Demos Stirling engine Thermoelectric engine Review: Second Law a. Kelvin-Plank b. new: “Clausius statement” Refrigerators (or air conditioners) heat, Qc fridge exhaust, Qh work COPrefrigerator: How good is your refrigerator? From warmup Why would you want to use a heat pump (instead of an electric heater) to heat your house when 100% of the energy in an electric heater can be converted directly to heat? Isn't that more efficient? Explain. a. No, because a heat pump is usually around 400% effective. That is, you get 4 times more energy (in the form of heat) out than you put in (in the form of work). Because it pulls energy from the air surrounding it, instead of just relying on the work that you put into it. Heat Pumps heat, Qc heat pump “exhaust”, Qh work COPheat pump: How good is your heat pump? “Reversible” vs. “Irreversible” “In order for a process to be [totally*] reversible, we must return the gas to its original state without changing the surroundings.” Warmup: Give an example of a process that would be considered reversible if not for that qualifier a. Yesterday at the physics social we froze things in liquid nitrogen. When a balloon went in, it compressed greatly. Then, when it was taken out, its volume expanded back to its original state. *Other terminology: internally reversible vs totally reversible. “Reversible” vs. “Irreversible” Warmup: Give an example of a process that would be considered reversible if not for that qualifier a. (My answer) Pretty much any line that you can draw on a PV diagram would be reversible if not for that condition. Consider a constant volume change, a vertical line on a PV diagram. If the surroundings are hot, the gas will move up the line towards higher temperature. By making the surroundings cold, the gas will move down the exact same line, reversing its path. P state B; TB = 650K state A; TA = 300K V From warmup The Carnot engine is completely impractical… Why then do we bother? What is important about this engine? a. It is important as a standard to measure the efficiency of other, more practical engines, and also as a measure of what is possible for humankind to achieve. Why doesn't the Carnot engine have perfect efficiency? a. Because no engine can have 100% efficiency b. Because that would require a cold reservoir at 0K, which is impossible. Carnot Cycle All heat added/subtracted reversibly a. During constant temperature processes b. Drawback: isothermal = slow, typically " eC " emax Tc 1 Th “C” for “Carnot” HW 11-5 – 11-7: find efficiency for a specific Carnot cycle Optional HW: eC derived for a general Carnot cycle Carnot Theorem Second Law, Kelvin-Plank statement a. You can’t fully convert heat to work b. You can’t have an efficiency of 100% Carnot Theorem: a. You can’t even have that! emax Tc eC 1 Th Th = max temp of cycle Tc = min temp of cycle Carnot Theorem: How to remember Engine: emax = ? Refrigerator: COPr,max = ? Heat pump: COPhp,max = ? Carnot Theorem: Proof Part 1 of proof: The Kelvin-Plank statement of the Second Law is equivalent to the Clausius statement. Clausius: Heat energy does not spontaneously flow from cold to hot. Kelvin-Plank: You can’t fully convert all heat to work. What if you could make heat go from coldhot? Then do this: work heat engine exhaust What if you could make a perfect engine? Then use it to power a refrigerator. Carnot Theorem: Proof Part 2 of proof: A totally reversible engine can be run backwards as a refrigerator. (Obvious? It’s really: “Only a totally reversible…”) Why not this? Bottom line: you could build a system to do that, but it couldn’t be built from an engine/heat reservoirs that look like this: P P V V Carnot Theorem: Proof Part 3 of proof: Suppose you had an engine with e > emax. Then build a Carnot engine using the same reservoirs, running in reverse (as a fridge). Use the fridge’s heat output to power the engine: work Qc work fridge Qh engine exhaust (at Tc) Which work is bigger? Can you see the problem? Multi-Stage Carnot Engine? Build a new cycle using only isotherms and adiabats. Result? “Regeneration” Any engineers in the crowd? The other way that you can transfer heat without changing entropy: internal heat transfer The Brayton cycle: Used by most non-steam power plants Isothermal contour Brayton cycle, cont. What does temperature look like at each point? Use “T-S” diagram. “S” = entropy, we’ll talk much more about on Monday For now, just know that adiabatic = constant S. Focus on y-axis Look here! Brayton cycle with regeneration Add another compressor & another turbine to increase the range over which regeneration can be done With an infinite number of compressors/turbines, you get the Carnot efficiency! (even with const. pressure sections) Image from http://web.me.unr.edu/me372/Spring2001/The%20Brayton%20Cycle%20with%20Regeneration.pdf (who apparently got it from a textbook, but I’m not sure which one)