Gas Laws
advertisement

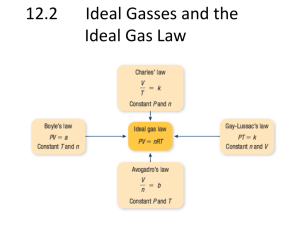
I. Physical Properties A. Kinetic Molecular Theory Particles in an ideal gas… • have no volume. • have elastic collisions. • are in constant, random, straightline motion. • don’t attract or repel each other. • have an avg. KE directly related to Kelvin temperature. C. Characteristics of Gases Gases expand to fill any container. • random motion, no attraction Gases are fluids (like liquids). • no attraction Gases have very low densities. • no volume = lots of empty space C. Characteristics of Gases Gases can be compressed. • no volume = lots of empty space Gases undergo diffusion & effusion. • random motion D. Temperature Always use absolute temperature (Kelvin) when working with gases. ºF -459 ºC -273 K 0 C F 32 5 9 32 212 0 100 273 373 K = ºC + 273 E. Pressure force pressure area Which shoes create the most pressure? E. Pressure Barometer • measures atmospheric pressure Aneroid Barometer Mercury Barometer E. Pressure Manometer • measures contained gas pressure U-tube Manometer Bourdon-tube gauge E. Pressure KEY UNITS AT SEA LEVEL 101.325 kPa (kilopascal) 1 atm 760 mm Hg 760 torr 14.7 psi N kPa 2 m F. STP STP Standard Temperature & Pressure 0°C 273 K -OR- 1 atm 101.325 kPa II. The Gas Laws BOYLES CHARLES GAYLUSSAC A. Boyle’s Law P Volume (mL) Pressure (torr) P·V (mL·torr) 10.0 20.0 30.0 40.0 760.0 379.6 253.2 191.0 7.60 x 103 7.59 x 103 7.60 x 103 7.64 x 103 PV = k V A. Boyle’s Law The pressure and volume of a gas are inversely related • at constant mass & temp P PV = k V B. Charles’ Law V T Volume (mL) Temperature (K) V/T (mL/K) 40.0 44.0 47.7 51.3 273.2 298.2 323.2 348.2 0.146 0.148 0.148 0.147 V k T B. Charles’ Law The volume and absolute temperature (K) of a gas are directly related • at constant mass & pressure V T V k T C. Gay-Lussac’s Law Temperature (K) Pressure (torr) P/T (torr/K) 248 273 298 373 691.6 760.0 828.4 1,041.2 2.79 2.78 2.78 2.79 P k T P T C. Gay-Lussac’s Law The pressure and absolute temperature (K) of a gas are directly related • at constant mass & volume P k T P T D. Combined Gas Law P V PV PV = k T P 1V 1 P 2V 2 = T1 T2 P 1 V 1T 2 = P 2V 2 T 1 E. Gas Law Problems gas occupies 473 cm3 at 36°C. Find its volume at 94°C. A CHARLES’ LAW GIVEN: T V V1 = 473 cm3 T1 = 36°C = 309K V2 = ? T2 = 94°C = 367K WORK: P1V1T2 = P2V2T1 (473 cm3)(367 K)=V2(309 K) V2 = 562 cm3 E. Gas Law Problems A gas occupies 100. mL at 150. kPa. Find its volume at 200. kPa. BOYLE’S LAW GIVEN: P V V1 = 100. mL P1 = 150. kPa V2 = ? P2 = 200. kPa WORK: P1V1T2 = P2V2T1 (150.kPa)(100.mL)=(200.kPa)V2 V2 = 75.0 mL E. Gas Law Problems gas occupies 7.84 cm3 at 71.8 kPa & 25°C. Find its volume at STP. A COMBINED GAS LAW GIVEN: P T V WORK: V1 = 7.84 cm3 P1V1T2 = P2V2T1 P1 = 71.8 kPa (71.8 kPa)(7.84 cm3)(273 K) T1 = 25°C = 298 K =(101.325 kPa) V2 (298 K) V2 = ? P2 = 101.325 kPa V2 = 5.09 cm3 T2 = 273 K