Document 14890203
advertisement
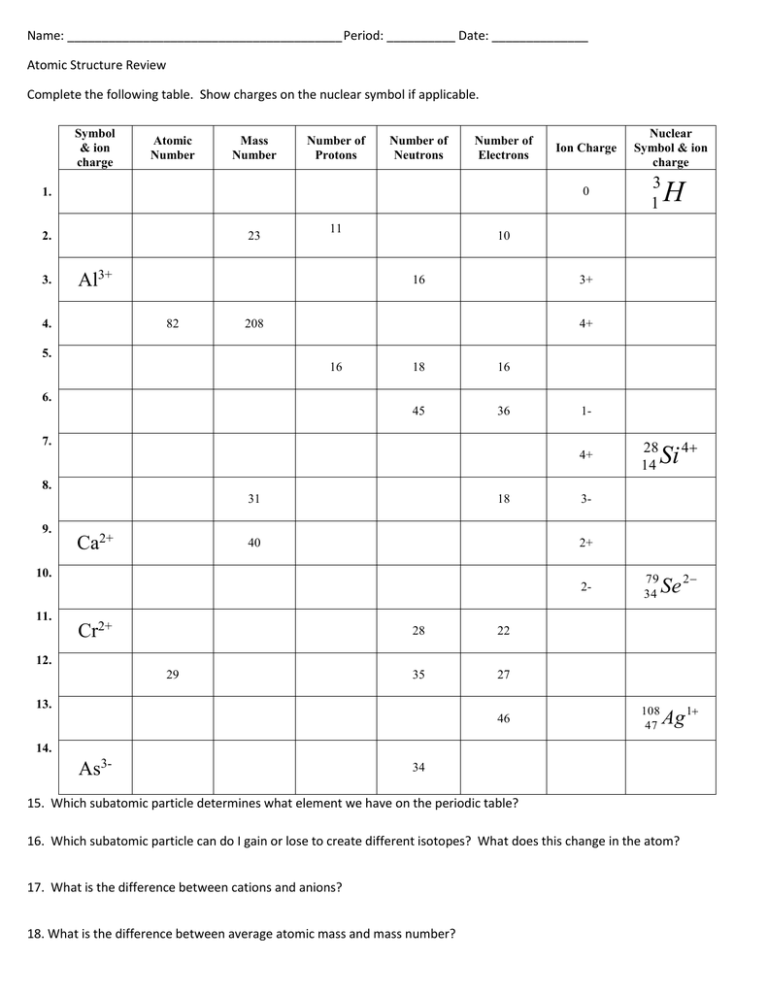
Name: ________________________________________ Period: __________ Date: ______________ Atomic Structure Review Complete the following table. Show charges on the nuclear symbol if applicable. Symbol & ion charge Atomic Number Mass Number Number of Protons Number of Neutrons Number of Electrons 0 1. 23 2. 3. Ion Charge 11 Al3+ 3 1 H 10 16 82 4. Nuclear Symbol & ion charge 3+ 208 4+ 5. 16 18 16 45 36 6. 1- 7. 4+ 28 14 Si 4 79 34 Se 2 108 47 Ag 1 8. 31 9. Ca2+ 18 40 3- 2+ 10. 211. Cr2+ 28 22 35 27 12. 29 13. 46 14. As3- 34 15. Which subatomic particle determines what element we have on the periodic table? 16. Which subatomic particle can do I gain or lose to create different isotopes? What does this change in the atom? 17. What is the difference between cations and anions? 18. What is the difference between average atomic mass and mass number? Average Atomic Mass: 1. The element lithium is composed of 5.90% 6Li and 94.10% 7Li. What is its average atomic mass? 2. Selenium has an average atomic mass of 78.957 amu, and has two isotopes. The first, Selenium – 77, has an isotopic mass of 77.012939 amu and a relative abundance of 19.91%. Find the mass of the second isotope. 3. Oxygen has three isotopes. The first, O-16, has a mass of 15.994914 amu and a percent abundance of 99.759. The second, O-17, has an isotopic mass of 16.999134 amu and occurs 0.0374% of the time. If oxygen has an average atomic mass of 15.9994 amu, what is the mass of the third isotope (O-18)? 4. The isotopes of plutonium occur according to the following chart. If the average atomic mass of uranium is 244.0 amu, what is the percent abundance of Pu – 239 and Pu- 246? Isotope Mass (amu) 239.5216 244.6419 246.3952 % Abundance X 39.54 Y 5. Einsteinium has two major isotopes, 255Es and 249Es. The atomic mass of einsteinium is 252.0 amu. What is the percent abundance of each isotope?







