Chemical Bonds
advertisement
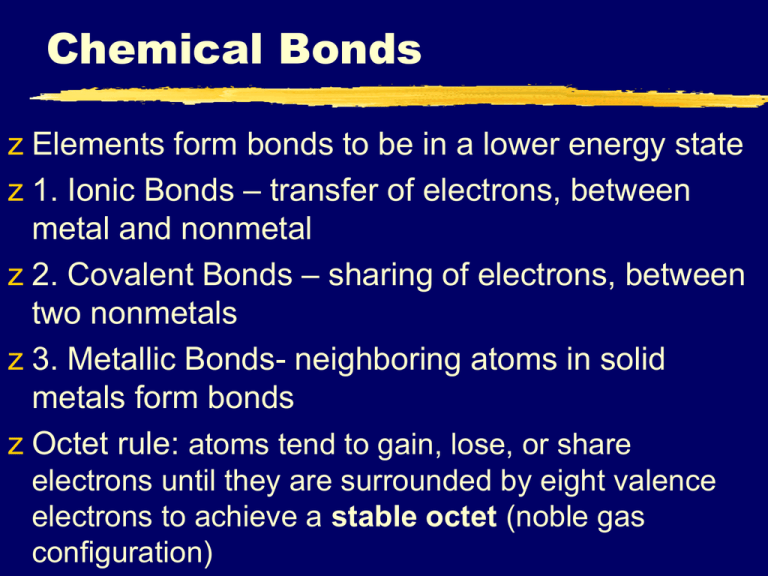
Chemical Bonds z Elements form bonds to be in a lower energy state z 1. Ionic Bonds – transfer of electrons, between metal and nonmetal z 2. Covalent Bonds – sharing of electrons, between two nonmetals z 3. Metallic Bonds- neighboring atoms in solid metals form bonds z Octet rule: atoms tend to gain, lose, or share electrons until they are surrounded by eight valence electrons to achieve a stable octet (noble gas configuration) Electron Dot Symbols z Valence electrons: reside in the highest occupied energy level, reside in the outer s & p orbitals and are the electrons involved in chemical bonding. z Electron-dot symbols are convenient way of showing the s & p electrons & tracking them in bond formation z -They consist of the chemical symbol for the element plus a dot for each valence electron z Consider sulfur whose electron configuration is [Ne]3s23p4, thus there are six valence electrons: S Ch. 7 – Ionic and Metallic Bonding I II III IV B. Ionic Bonds • Ionic Bonds – atoms transfer electrons from a cation (positive ion) to an anion (negative ion) to achieve an octet. • Ionic compounds are stable due to the electrostatic forces between unlike charges organizing the ions of ionic substances into a rigid, organized three-dimensional arrangement: • The ions are drawn together • Energy is released • Ions form solid lattice structure Ionic Rule 1: Metals with a Single Oxidation Number Bound to Non-Metals The metal will take its positive oxidation number and the non-metal will have to take its negative oxidation number. Only one compound can be formed Name of Metal ( Root of Non-metal ) -ide Ionic Rule 1: Metals with a Single Oxidation Number Bound to Non-Metals Example 1: Sodium reacts with oxygen to produce Na2O, what is the name of this compound Since there is only one possible compound, we do not have to indicate the number of elements sodium oxide Ionic Rule 1: Metals with a Single Oxidation Number Bound to Non-Metals Example 2: What is the chemical formula for aluminum oxide First write the symbols of the elements Next write the oxidation number of each element above that element Switch the oxidation numbers and reduce 3 -2 2 Al O Comprehension Check What is the name of Mg3N2? magnesium nitride What is the name of Li2Se? lithium selenide What is the formula for indium chloride? InCl3 What is the formula for potassium phosphide? K3P Ionic Rule 2: Metals with Multiple Oxidation Numbers Bound to Non-Metals The metal will take one of its positive oxidation numbers and the non-metal will have to take its negative oxidation number. Since the metal has more than one possible oxidation number, multiple compounds can be formed We need a distinct name for each Name of Metal ( Metal’s Oxidation State as a Roman Numeral )( Root of Non-metal ) -ide Ionic Rule 2: Metals with Multiple Oxidation Numbers Bound to Non-Metals Example 1: What is the name of IrBr6? First we need to determine how many electrons that iridium needs to lose in order to satisfy 6 bromine atoms. Each bromine needs one electron There is only one iridium in this compound Therefore, the iridium atom will have to supply all six electrons, giving it a +6 oxidation number. iridium(VI) bromide Ionic Rule 2: Metals with Multiple Oxidation Numbers Bound to Non-Metals Example 2: What is the formula for mercury(II) nitride? First write the symbols of the elements Next write the oxidation number of each element above that element Switch the oxidation numbers and reduce 2 -3 3 Hg N Comprehension Check What is the name of RuN? ruthenium(III) nitride What is the name of MnO3 manganese(VI) oxide What is the formula for paladium(IV) bromide? PdBr4 What is the formula for molybdenum(V) sulfide? Mo2S5 Ionic Rule 3: Metals with a single Oxidation Number Bound to Polyatomic Ions Polyatomic Ions – strongly bound group of atoms that have either lost or gained electrons and become charged. List of common Polyatomic Ions are on the back of your Periodic Table Polyatomic ions act as a single atom, with a single name Subscripts within the ion cannot be changed Since there is only one oxidation number for the metals and Polyatomic Ion, only one compound can be produced. Ionic Rule 3 : Metals with a single Oxidation Number Bound to Polyatomic Ions Naming these compounds is just like rule 1, except we do not add –ide to the end of the polyatomic ion Name of Metal ( Name of Polyatomic Ion ) Ionic Rule 3 : Metals with a single Oxidation Number Bound to Polyatomic Ions What is the name of Mg(NO3)2 First, you should recognize that there are more than two elements involved, which means that a Polyatomic Ion is involved Next, look up the Metal in the periodic table and confirm that it has a single oxidation number Look up the name of the Polyatomic Ion magnesium nitrate Ionic Rule 3 : Metals with a single Oxidation Number Bound to Polyatomic Ions What is the formula for calcium iodite? First, since the second name does not end in –ide, a polyatomic ion is involved. Write the symbol for calcium and formula for iodite. Write the oxidation numbers above the metal and the polyatomic ion Switch the numbers, and use parenthesis around the polyatomic ion if necessary 2 -1 1 Ca (IO2 ) Comprehension Check What is the name of KHSO4? potassium hydrogen sulfate potassium bisulfate What is the name of In2(C2O4)3? indium oxalate What is the formula of strontium bromate? Sr(BrO3)2 What is the formula for germanium phosphate? Ge3(PO4)4 Ionic Rule 4: Metals with Multiple Oxidation Numbers Bound to Polyatomic Ions When the metal has more than one possible oxidation number, more than one compound can be formed We must use Roman Numerals to indicate which oxidation number the metal is using Name of Metal ( Metal’s Oxidation State Name of as a Roman Numeral Polyatomic Ion ) Ionic Rule 4 : Metals with Multiple Oxidation Numbers Bound to Polyatomic Ions What is the name of RhSO4? First, there are more than two elements involved Look up the oxidation and name of SO4 Sulfate (-2) Finally, figure out which oxidation number the metal is using. There is only one rhodium, so it must account for all of the electrons & would have to take a +2 oxidation number rhodium (II) sulfate Ionic Rule 4 : Metals with Multiple Oxidation Numbers Bound to Polyatomic Ions What is the formula for nickel(II) ferrocyanide? First, since the second name does not end in -ide, a polyatomic ion is involved Write the symbol for nickel and formula for ferrocyanide Write the oxidation numbers above the metal and the polyatomic ion Switch the numbers, and use parenthesis around the polyatomic ion if necessary and reduce 2 -4 4 Ni 2(Fe(CN)6) Comprehension Check What is the name of Cr(IO)3? chromium(III) hypoiodite What is the name of CuMnO4? copper(II) manganate or copper(I) permanganate What is the formula for palladium(IV) ferricyanide? Pd3(Fe(CN)6)4 What is the formula for molybdenum(VI) dichormate? Mo(Cr2O7)3 B. Properties of Ionic Compounds • Most ionic compounds are crystalline solids at room temperature • Arranged in repeating threedimensional patterns • Ionic compounds generally have high melting points • Large attractive forces result in very stable structures B. Properties of Ionic Compounds • Ionic compounds can conduct an electric current when melted or dissolved in water • When ionic compounds are dissolved in water the crystalline structure breaks down so ions are able to move freely which results in conductivity Ch. 7 – Ionic and Metallic Bonding III. Bonding in Metals (p. 201 – 203) I II III IV A. Metallic Bonding Metallic bonds: Consist entirely of metal atoms. • Bonding is due to valence electrons which are delocalized throughout the entire solid • The metal is held together by the strong forces of attraction between the positive nuclei and the delocalized electrons. B. Metals • Metals are good conductors of heat and electricity because the valence electrons are able to flow freely • Valence electrons of metals can be thought of as a ‘sea of electrons’, very mobile C. Metallic Bond Metallic Bonding - “Electron Sea” D. Metallic Properties • Have luster, are ductile and malleable • Luster = shine • Ductile = ability to be drawn into wires • Malleable = ability to be shaped, pounded, etc D. Metallic Properties • Properties can be explained by the mobility of electrons in metals • When subjected to pressure , the cations easily slide past each other like a ball bearing immersed in oil. Covalent Bonding What is a covalent bond? • A chemical bond that results from the sharing of electrons, to form a stable octet or duet (Hydrogen only needs 2 to be stable) • Molecule = two or more atoms that are held together by covalent bonds H2O • Majority of covalent bonds form between nonmetals (CLOSE together on periodic table) Covalent Rule 1: Nonmetals Bound to Nonmetals • Since nonmetals have more than one oxidation number, there will always be more than one compound produced – Therefore we have to have a distinct name for each compound – To do this we use a prefix to indicate how many atoms of each element are present One – mono- Five – penta- Two – di- Six – hexa- Three – tri- Seven – hepta- Four – tetra- Eight – octa- Nine – nona Ten – deca Covalent Rule 1: Nonmetals Bound to Nonmetals Prefix + Nonmetal prefix + (root of nonmetal) -ide • Using prefixes – The prefix mono- is only used on the second element • Ex: PF3 is named phosphorus trifluoride – If two vowels are adjacent, leave them • Ex: NI3 is named nitrogen triiodide – In the case of monoxide only, drop one “o” – Cannot reduce subscripts on covalent Naming! Covalent Rule 1: Nonmetals Bound to Nonmetals • Ex 1: What is the name of P2S3? – diphosphorus trisulfide • Ex 2: What is the name of As7I3? – heptaarsenic triiodide • Ex 3: What is the chemical formula of dihydrogen monoxide? – H2O • Ex 4: What is the chemical formula of dinitrogen pentaoxide? – N2O5 Covalent Bonding Formation • Diatomic molecule – molecule containing the same two atoms • Some elements always exist this way because they are more stable than the individual atoms Cl2 B. Diatomic Elements • The Seven Diatomic Elements Br2 I2 N2 Cl2 H2 O2 F2 H N O F Cl Br I Bonds in all the polyatomic ions and diatomics are all covalent bonds Single Covalent Bonds Two atoms held together by a sharing of one pair of electrons are joined together by a single covalent bond. Single Covalent Bonds An electron dot structure represents the shared pair of electrons of the covalent bond by two dots. A structural formula represents the covalent bonds by dashes and shows the arrangement of covalently bonded atoms Single Covalent Bonds A pair of valence electrons that is not shared between atoms is called an unshared pair, also known as a lone pair of a nonbonding pair. Lone pair Double and Triple Covalent Bonds Atoms form double or triple covalent bonds if they can attain a noble gas structure by sharing two or three pairs of electrons. A double bond involves sharing two pairs of electrons. A triple bond involves sharing three pairs of electrons. Double and Triple Covalent Bonds Molecular Structure Lewis Diagrams (p. 220 – 229) I II III Drawing Lewis Diagrams 1. Arrange atoms • • • Singular atom is usually in the center (often Carbon) If not Carbon, least e- neg atom is in center Hydrogen is always terminal 2. Find total # of e- available to bond (valence e- ) 3. Place a pair of electrons between central atom and each terminal atom Drawing Lewis Diagrams 4. Place remaining electrons in pairs around terminal atoms (except H) to satisfy octet rule • Any remaining pairs are assigned to central atom 5. Determine whether or not central atom satisfies octet • If not, convert one or more lone pairs from terminal atoms to double or triple bonds • Certain atoms can be exceptions to octet rule – H, Be, B, S, P, Xe Drawing Lewis Diagrams CF4 1 C × 4e- = 4e4 F × 7e- = 28e32e- 8e24e- F F C F F Drawing Lewis Diagrams CO2 1 C × 4e- = 4e2 O × 6e- = 12e16e- 4e12e- O C O Polyatomic Ions To find total # of valence e-: Add 1e- for each negative charge Subtract 1e- for each positive charge Place brackets around the ion and label the charge Polyatomic Ions ClO41 Cl × 7e- = 7e4 O × 6e- = 24e31e+ 1e32e- 8e24e- O O Cl O O Octet Rule Exceptions: F F F B F Boron & Beryllium get 6 & 4 valence e H O H respectively F SF F Expanded octet more than 8 valence Fe (e.g. S, P,FXe) Hydrogen 2 valence e- - - Resonance Structures Molecules that can’t be correctly represented by a single Lewis diagram Actual structure is an average of all the possibilities Show all possible structures separated by double-headed arrows C. Resonance Structures SO3 O O S O O O S O O O S O Bond Polarity • Most bonds are a blend of ionic and covalent characteristics. • Difference in electronegativity determines bond type. Bond Polarity • Electronegativity – Attraction an atom has for a shared pair of electrons. – higher e-neg atom – lower e-neg atom + Electronegativity Difference • If the difference in electronegativities is between: – 1.7 to 4.0: Ionic – Greater than 0.3 & less than 1.7: Polar Covalent – 0.0 to 0.3: Non-Polar Covalent The type of bond can usually be calculated by finding the difference in electronegativity of the two atoms that are bonded. Compound F2 or F-F CF4 LiF or Li-F Electronegativity 4.0 - 4.0 = 0 4.0 - 2.5 = 1.5 4.0 - 1.0 = 3.0 Difference Non-polar (strong) Polar Ionic (nonType of Bond covalent covalent covalent) Bond Polarity • Nonpolar Covalent Bond – e- are shared equally – symmetrical e- density – usually identical atoms • Ex: H2 or Cl2 Bond Polarity • Polar Covalent Bond – e- are shared unequally – asymmetrical e- density – results in partial charges (dipole) • Ex: H2O + Ch. 8 – Molecular Structure Molecular Geometry (p. 232 – 236) I II III VSEPR Theory Valence Shell Electron Pair Repulsion Theory Electron pairs orient themselves in order to minimize repulsive forces VSEPR Theory Types of e- Pairs Bonding pairs – form bonds Lone pairs – nonbonding e Total e- pairs– bonding + lone pairs Lone pairs repel more strongly than bonding pairs!!! A. VSEPR Theory Lone pairs reduce the bond angle between atoms Bond Angle Determining Molecular Shape Draw the Lewis Diagram Tally up e- pairs on central atom (bonds + lone pairs) double/triple bonds = ONE pair Shape is determined by the # of bonding pairs and lone pairs Common Molecular Shapes 2 total → Electronic Geometry = linear 2 bond 0 lone BeH2 LINEAR 180° Common Molecular Shapes 3 total → Electronic Geometry = trigonal planar 3 bond 0 lone BF3 TRIGONAL PLANAR 120° Molecular Polarity Polar molecule = one end slightly + and one end slightly – Molecule with 2 poles = dipolar molecule or dipole Molecular Polarity Shape, symmetry and bond polarity determines molecular polarity H – O bond is polar and water is asymmetrical, so H2O is polar C – Cl bond is polar, but CCl4 is symmetrical, so molecule is nonpolar Molecular Polarity Identify each molecule as polar or nonpolar • O2 Nonpolar bonds → nonpolar • CS2 Nonpolar bonds → Linear → nonpolar Polar bonds → Tetrahedral → nonpolar • CF4 • H2O Polar bonds → Bent → polar I. Intermolecular Forces Intramolecular and Intermolecular Forces Covalent & Ionic bonds - the force that holds atoms together making molecules. • These are intramolecular forces. There are also forces that cause molecules to attract each other. These are called intermolecular forces. Definition of IMF Attractive forces between molecules. Much weaker than chemical bonds within molecules. a.k.a. van der Waals forces Types of IMF