A Common Mechanism of Cellular Death Induced by Bactericidal Antibiotics
advertisement

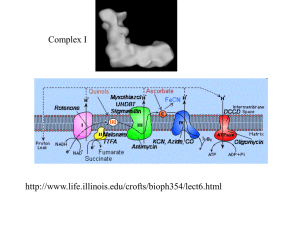
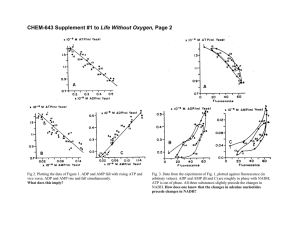
A Common Mechanism of Cellular Death Induced by Bactericidal Antibiotics Michael A. Kohanski, Daniel J. Dwyer, Boris Hayete, Carolyn A. Lawrence, and James J. Collins Boston University, Boston, MA Cell. 2007 Sept 7;130( 5): 797-810 Two Anti-Microbial Therapies • Bactericidal – Kill >99.9% bacteria – Specific Drug-Target interactions • Bacteriostatic – Inhibit growth of bacteria • Sometimes not a clear line between two classes. High concentrations of bacteriostatic can result in killing. Classes of Drug-Target interactions (Bactericidal) • Inhibit DNA replication/repair – Quinolines (NORFLOXACIN) • Inhibit Protein synthesis – Aminoglycosides (KANAMYCIN) • Inhibit Cell-wall turnover – β-lactams (AMPICILLIN) Question: What is the bacteria’s response to this primary drug-target interaction? Bacteriostatic • Target 30S ribosome function – Ex: TETRACYCLINE, SPECTINOMYCIN • Target 50S ribosome function – Ex: CHLORAMPHENICOL, ERYTHROMYCIN • Target RNA polymerase – Ex: RIFAMYCIN Fenton Reaction H2O2 + Fe2+ Fe3+ + OH* + OH- Question: What is the source of Fe? Ex: Intracellular- Fe-S clusters Extracellular- Iron Import Previously, Dwyer et al. showed that gyrase inhibitors (quinoline synthetic antibiotics) induce breakdown in Fe regulation, which leads to OH* formation Question: * Does OH formation contribute to antibiotic-induced cell death and are there differences among classes of antibiotics? Flow Cytometry and HPF OH* OH* Argon FIGURE 1: E. coli Cell Death by H2O2 Hours (post-treatment) HPF Fluorescence Log Fluor Intensity Thiourea: OH* scavenger (sequesters OH*) Dipyridyl: Iron Chelator (sequesters unbound Fe) Conclusion: HPF good at measuring hydroxyl radical formation Amp addition Cell Death by Bactericidals FIGURE 1: E. coli HPF Fluorescence Note: Bimodal curve Amp treated Conclusion: Bactericidal antibiotics promote formation of OH* Similar results with Gram+ Staphylococcus aureus Seems killing of cells correlates to OH* production What about lethal doses of bacteriostatic drugs? Is OH* formation important in bactericidal killing? Figure 2 My question: H2O2 + thiourea = NO OH* as seen by HPF Norfloxacin + thiourea = some OH* production... Why the difference? Fenton Reaction H2O2 + Fe2+ Fe3+ + OH* + OH- Where is this iron coming from? Iron transport/import? TonB mutant Structure of TonB in Complex with FhuA, E. coli Outer Membrane Receptor P.D. Pawelek1, N. Croteau1, C. Ng-Thow-Hing1, C.M. Khursigara1, N. Moiseeva2, M. Allaire2 and J.W. Coulton1 1Department of Microbiology and Immunology, McGill University, 2National Synchrotron Light Source, Brookhaven National Laboratory tonB no protective effect from bactericidals Conclusion: Fe in Fenton reaction not from extracellular source Fenton Reaction H2O2 + Fe2+ Fe3+ + OH* + OH- Where is this iron coming from? Fe-S clusters? IscS mutant Lauhon, C. T. et al. J. Biol. Chem. 2004;279:19551-19558 Fig 2 (again) Conclusion: Fe in Fenton reaction from intracellular source (Fe-S) Table 1: aka the largest table EVER Microarray data of genes regulated by bactericidal drug classes relative to spectinomycin • Upregulated (38) – NADH Dehydrogenase ONLY pathway upregulated by all three! • Downregulated (142) – β-lactam resistance membrane protein – Multidrug efflux system transporter – Transport, drug/analog sensitivity • Why spectinomycin? Fe leaching via superoxides And how do we get superoxides? Oxidative Phosphorylation Complex I Complex III H+ Complex IV ATPase H+ H+ H+ cyt C Q nuo NUO ee-- H+ H+ NADH NAD+ NADH NAD+ Cyt bc1 cyt bc1 cyt aa3 cbb3 1/2 O2 O2 H+ H2O O2• ADP + Pi ATP H+ Figure 3A: Nad+ Cycling Assay Collect culture, centrifuge, flash freeze pellet Add NaOH-NADH extraction, HCl-NAD+ extraction (stability) Boil 10 min, centrifuge, collect supernatant = sample 96 well plate: sample, bicine, neutralizing buffer, phenazine ethosulfate (ecarrier), MTT (redox indicator), EtOH (dissolves MTT), EDTA Add yeast alcohol dehydrogenase to start reaction-kinetics 570nm Rate of MTT reduction is proportional to conc of NAD+ or NADH NAD+, NADH standards for calibration Figure 3: Decreased NADH pool leads to decreased O2 leads to less Fe leaching leads to less OH* leads to less death Repressor SOS SOS RESPONSE GENES DNA Damage Activates RecA Promotes LexA auto cleavage SOS RESPONSE GENES AMP GF P Measure Fluorescence pZ vector O ri Figure 4 Note: Norfloxacin induces SOS Bimodal Amp curve -Corresponds with lysis No Kan difference -Requires transcription AND translation of GFP -Kan disrupts translation Figure 5: RecA mutant Bactericidal Drug Target not important Upregulation of NADH Dehydrogenase Rapid depletion of NADH O2* Destabilize Fe from Fe-S clusters Figure 6: Big Picture FENTON REACTION! Hydroxyl Radicals! DEATH! Final Comments?