WASTE CONTAINMENT TECHNOLOGY Dr. Grace Hsuan Civil & Architectural Engineering
advertisement

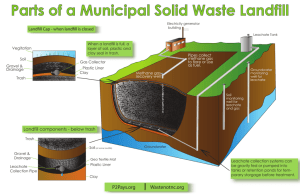
WASTE CONTAINMENT TECHNOLOGY Dr. Grace Hsuan Civil & Architectural Engineering Outlines • Waste management methods • Landfill design and regulations • Function and usage of geosynthetics in landfill systems • Durability of geosynthetics • Future trend of landfill management Waste Classification • • • • • Municipal waste Construction demolition debris Nonhazardous industrial waste Incineration ash Hazardous waste Amount of Municipal Waste Million of Tons 200 150 100 50 0 1950 1960 1970 1980 Year 1990 2000 Waste Management Methods Method 1970 1980 1990 Landfilling 72% 81% 67% 1992 (Goal) 55% Combustion 21% 9% 16% 20% Recycling 7% 10% 17% 25% Source Reduction Source reduction involves reduction in the quantity or toxicity of materials during the manufacturing process via: • Decrease the amount of unqualified products by improving quality control • Decrease the unit weight of the product by using high quality material. Weight Reduction (unit of grams) Container 1980 1992 2-liter PET bottle 65 51 Aluminum can 19 15 Glass soda bottle 255 177 Steel (tin) soup can 48 37 Half-pint milk carton 14 11 Recycling Materials in Percentage of Waste Materials Corrugated boxes Newspapers Office paper Glass containers Steel cans Aluminum cans Plastics packaging Yard waste Others Total 1985 1990 Projected 1992 4.4 5.9 6.7 2.1 2.8 3.3 0.7 0.9 1.0 0.7 1.3 1.5 0.1 0.2 0.2 0.4 0.5 0.5 0.1 0.2 0.2 0 2.2 2.7 1.5 3.0 3.8 10 17.1 20.2 Combustion • Combustion can reduce the volume of the solid waste up to 90% at the same generate power. • There are 140 combustion plants the US. • Emission must meet the EPA Clean Air Act. • Residual ash is hazardous material and should be disposed accordingly. Landfill • Landfill implies disposal of waste in the ground. • 70% of the waste is disposed in landfill and the percentage has been gradually decreasing. • The amount of waste actually increased due to population growth. Landfilling Approximately 6,500 landfills operate in the US: – 57% belong to local governments – 14% belong to private companies, – 29% belong to federal agencies or solid waste authorities. Landfill Capacity The size and capacity vary greatly: • 30% of the landfills receive less than 30 tons per day • 5% receive more than 500 tons The Largest Landfill • • • • Fresh Kills, Staten Island, NY 3,000 acres 2.4 billion cubic feet of waste • 25 times of the great pyramid Nature of Waste Problem Moisture within and flowing on the waste generates leachate Leachate takes the characteristics of the waste Thus leachate is very variable and is sitespecific - there is no "typical" leachate Flows gravitationally downward into the leachate collection system Enters groundwater unless a suitable barrier layer or system is provided Current Legislation • EPA for both non-hazardous and hazardous waste • Superfund via Corps of Engineers • DOE/NRC for radioactive wastes • Worldwide approx. 40 countries have legislation/regulations (survey in GRI Report #23) Regulations Solid waste is regulated under the Resource Conservation and Recovery Act (RCRA). Classification of non-hazardous and hazardous waste depends on the chemical constituents of the leachate. Hazardous Waste Definition • Waste is listed in Appendix VIII of Title 40, Code of Federal Regulations, Part 251. • Waste is mixed with or derived from hazardous waste. • Waste is not identified as municipal waste. • Waste possesses one of the following characteristics: – ignitable; corrosive; reactive and toxic. Minimum Technology Guidance (MTG) • Federal regulation on landfill design requirement is published by the EPA. • Dependent on the classification of the waste, MTG is recommended. • Each state must follows, or exceed, the MTG. Non-hazardous Waste • Non-hazardous waste is regulated under Subtitle D of RCRA. • EPA regulations are published in Parts 257 and 258, Title 40, Code of Federal Regulations (CFR). Minimum Technology Guidance (MTG) for a Subtitle D Landfill “Solid Waste” Composite liner 150 mm Filter (or GT) 300 mm Drain (or GN/GC) GT (opt.) GM* 600 mm Clay @ 1x10-7 cm/sec Soil Subgrade Hazardous Waste • Hazardous waste is regulated under Subtitle C of RCRA. • EPA regulations are published in Part 264.221, Title 40, Code of Federal Regulations (CFR). MTG for a Subtitle C Landfill “Solid Waste” 150 mm Filter (or GT) 300 mm Drain (or GN/GC) P-GM* 300 mm Drain (or GN) S-GM* Composite liner 900 mm Clay @ 1x10-7 cm/sec 3.0 m (to highest groundwater level) Landfill Closure Activities • Closure must begin within 30 days of final receipt of waste; extensions may be granted by state approval. • Closure must be completed in accordance with closure plan within 180 days; extensions may be granted by state approval. • A notation must be placed in the deed. Landfill Covers (Non-hazardous landfill without Geosynthetic on the bottom liner system) 150 mm 450 mm Erosion Layer Infiltration Layer Cover Layers • Erosion Layer – Earthen material is capable of sustaining native plant growth • Infiltration Layer – Permeability of this layer of soil should be less than or equal to the permeability of any bottom liner system or natural subsoils present, or permeability less than 1x10-5 cm/sec whichever is less Landfill Cover System (Subtitle C & D, and Corp of Eng.) 150 mm Varies (frost depth) Topsoil Cover Soil 150 mm Filter (or GT) 300 mm Drain (or GN) GM 600 to 900 mm Clay @ 1x10-7 cm/sec 300 mm Gas Vent (or GT) ”Solid Waste” Landfill Site • Conforms with land use planning of the area • Easy access to vehicles during the operation of the landfill • Adequate quantity of earth cover material that is easily handled and compacted • Landfill operation will not detrimentally impact surrounding environment • Large enough to hold community waste for some time Geosynthetics geomembranes (GM) geosynthetic clay liners (GCL) geonets (GN) geotextiles (GT) geogrids (GG) geopipe (GP) geocomposites (GC) Primary Functions Type GM GCL GN GT GG GP GC S Y Y R Y Y Y F Y Y D Y Y Y Y S = separation, R = reinforcement, F = filtration D = drainage, B = barrier B Y Y Y Natural Soils vs. GSs Function Natural Soil Barrier-Single CCL BarrierComposite GM/CCL Geosynthetics GM GM/GCL GM/GCL/CCL Drainage Layer Sand GT Gravel or sand GN Filter Layer Sand GT Liner System GT GN GCL GM GG CCL Gravel w/ perforated pipe Final Cover System Solid Waste Possible Geosynthetic Layers in a Waste Containment System in Final Cover - 7 in Base Liner - 9 16 Layers! Liquid Barrier Systems • Single CCL • Single GM • Single composite liner – GM/CCL • Double composite liner – GM/CCL-GM/CCL – GM/GCL-GM/CCL Composite Barriers (Intimate Contact Issue) Leachate Leachate CCL CCL Clay Liner Composite Liner (by itself) (with intimate contact) Leachate GCL Composite Liner (GM + GCL) Does the GT compromise the composite liner concept? Ans: Generally no... Composite Barriers (Theoretical Leakage) GM alone (hole area “a”) Composite liner (GM/CCL) Leachate ks Q = CB a 2 gh Q = 0.21 a0.1 h0.9 ks0.74 Q= (for good contact) 1.15 a0.1 h0.9 ks0.74 (for poor contact) Ref. Bonaparte, Giroud & Gross, GS ‘89) Generalized Leakage Rates Through Liners (ref. Giroud and Bonaparte, Jour. G & G, 1989) Type of Liner Geomembrane alone (between two sand layers) Composite liner (poor field conditions, i.e., waves) Composite liner (good field conditions, i.e., flat) Leakage Mechanism Diffusion Small holes* Large holes* Diffusion Small holes* Large holes* Liquid height on top of the geomembrane 0.03 m 0.3 m 3m 30 m 0.01 1 10 300 300 1,000 3,000 10,000 10,000 30,000 100,000 300,000 0.01 1 100 300 0.8 6 50 400 1 7 60 500 Diffusion Small holes* Large holes* 0.01 1 100 300 0.15 1 9 75 0.2 1.5 11 85 Values of leakage rate in lphd (figures to be divided by approximately 10 to obtain values expressed in gpad) *assumes 3 holes/ha (i.e., 1.0 hole/acre) Response Action Plans (RAP's) • Only applicable with double liner systems • Worldwide, 58% HSW (incl. USA) and 14% of MSW require double liner systems • Requires measurement of liquid quantity in leak detection system • If above the preset action leakage rate (ALR), different requirements are set in motion, e.g., – – – – continuous monitoring characterize liquid stop receiving waste remove waste to locate leak(s) Some Comments on RAP's (a) "de minimum" leakage ~ 10 lphd (~ 1.0 gpad) vapor diffusion through perfect geomembrane with no flaws = 0.2 to 20 lphd (b) typ. action leakage rate (ALR) ~ 50 to 200 continuous monitoring assess liquid characteristics compare to primary leachate (c) typ. intermediate leakage rate (ILR) ~ 200 to 1000 stop adding waste continue monitoring and testing (d) typ. rapid and large leak (RLL) > 1000 lphd remove waste repair leak(s) Note: all of the above RAP values are for illustration only -- they must be site specifically determined -- note that EPA only requires the establishment of an ALR value Average Values of Leakage Quantities Leakage Rate (lphd) 40 Sand Leak Detection 30 GM 20 GM/CCL 10 GM/GCL 0 1 2 Life Cycle Stage 3 Average Values of Leakage Quantities (cont’d) Leakage Rate (lphad) 20 Geonet Leak Detection 15 GM/CCL 10 GM 5 GM/GCL 0 1 2 Life Cycle Stage 3 Geomembranes Widely Used Geomembranes Limited Used Geomembranes High density polyethylene (HDPE) Chlorosulfonated polyethylene (CSPE) Linear low density polyethylene (LLDPE) Ethylene interpolymer alloy (EIA) Flexible polypropylene Ethylene propylene trimonomer (EPDM) (f-PP) Polyvinyl chloride-plasticized (PVC-p) Comments • • • • Name is associated with resin type All have some amount of additives Additives can vary from 2% to 60% Some additives are critical to performance Compositions (approximate percentage) Type Resin Plasticizer Antioxidant Filler 95-97 Carbon Black 2-3 HDPE 0 1-0.5 0 LLDPE PVC-p fPP 95-97 50-70 95-97 2-3 1-2 2-3 0 25-35 0 1-0.5 1-0.5 1-0.5 0 5-10 0 CSPE 40-60 5-40 0 1-0.5 5-15 EPDM 25-30 20-40 0 1-0.5 20-40 Geomembrane Styles • smooth geomembranes • Textured geomembranes • Reinforced geomembranes Manufacturing Processes • • • • Flat extrusion Blown sheet extrusion Blown sheet co-extrusion Calendaring Material Properties • • • • • • Mechanical property Density Melt flow Carbon black Plasticizers Antioxidant Tensile Behavior • Test method varies according to the resin type and style of the geomembrane. • Each test method consists of unique shape of specimen and strain rate. • Methods: – HDPE, LLDPE and fPP – ASTM D 638 Type IV – PVC-p – ASTM D 882 – All reinforced geomembranes – ASTM D 751 Design Concept Allowable (Test) Property FS = Required (Design) Property Where: • Test methods are from ASTM, ISO, or others • Design models from the literatures • Factor-of-Safety is site specific Density Methods • ASTM D 752 (Specific gravity) • ASTM D 1505 (Density column) • ASTM D 4883 (Ultrasonic for PE only) HDPE Geomembranes • Resin density is around 0.930 g/cc, which is in the medium density range according to ASTM D 833. • The 2.5% carbon black raise the density of the product to 0.941g/cc, which is the HDPE range. product = resin + 0.0044C Where: C = weight percentage of carbon black Melt Flow (MI) Method • • • • Test Method - ASTM D 1238 Only for thermoplastic materials Test condition varies with resin type It is essential for extrusion process, i.e., for product manufacturers • For the same type of polymer, MI can be correlated to the molecular weight Function of Carbon Black • The primary function is as an ultraviolet light stabilizer to protect polymer being degraded. • Carbon black absorption coefficient increases with loading up to ~ 3%. • In elastomeric materials, carbon black also functions as an reinforcement, and loading can be as high as 30-40%. Addition of Carbon Black • The masterbatch technique is utilize to dispersing carbon black in plastic. • A masterbatch is a resin containing a high concentration of carbon black. • The masterbatch is blended with polymer resin to achieve the desire percentage. Carbon Black • Carbon black content is measured according to ASTM D1603. • Carbon black dispersion is evaluated according to ASTM D 5596. Plasticizers • Plasticizers is used in PVC to lower the glass transition temperature (Tg). • An addition of 30% plasticizer in PVC can lower the Tg from 80oC to –20oC. • The plasticized PVC behaves rubbery at normal ambient temperature. • However, plasticizer can slowly leach out with time. Analysis Plasticizers • The amount of plasticizer in the polymer can be determine by extraction according to ASTM D 2124. • The type of plasticizer can be identified using Infrared (IR) spectroscopic. Antioxidants • The function of antioxidants is to protect polymers from being oxidized during the extrusion process and service lifetime. • For polyolefines, antioxidants is vital to the longevity of the product. • Antioxidant will be the focus of the second part of this class. Degradation of HDPE Geomembranes Chemical Related: – Thermal-oxidation – Photo-oxidation Linear PE Structure • Linear PE is a graft copolymer • Each co-monomer creates one branch • Co-monomer can be butene, hexene, or octene Density of Geomembranes • Density decreases as the amount of co-monomer increases • Density range of PE (ASTM D883) – > 0.940 g/ml for HDPE – 0.926 - 0.940 g/ml for MDPE – 0.910 - 0.925 g/ml for LLDPE – <0.909 g/ml for VLDPE or ULDPE II. Oxidation Degradation • Polyolefins, such as HDPE, PP and PB are susceptible to oxidation. • Oxidation takes place via free radical reactions. • Free radicals form at the tertiary carbon atoms (i.e., at branches). • Oxidation leads to chain scission that results in decrease of Mw and subsequently on mechanical properties. Forming Free Radicals Different Degradation Stages Various Stages of Oxidation Reactions during Induction Period RH R H R O2 ROO ROO RH ROOH R Reactions during Acceleration Period OH RH H 2 O R ROOH RO OH RO RH ROH R Functions of Antioxidants • Primary antioxidants react with free radical species • Secondary antioxidants decompose ROOH to prevent formation of free radicals Types of Antioxidants Category Primary Secondary Chemical Type Example Hindered phenol Irganox 1076 or 1010 Santowhite crystals Hindered amines Various of Tinuvin, Chemassorb 944 Phosphites Irgafos 168 Sulfur compound Dilauryl thiodipropionate Distearyl thiodipropionate Hindered amines Various of Tinuvin, Chemassorb 944 Effective Temperature Range Phosphites Hindered Phenols Thiosynergists Hindered Amines 0 50 100 150 200 Temperature (oC) 250 300 Depletion of Antioxidants Two mechanisms: a. Chemical reactions – by reacting with free radicals and peroxides b. Physical loss – by extraction or volatilization Arrhenius Model Rate of reaction = X * Y * Z Where: X = collision frequency (concentration or pressure) Y = energy factor Z = probability factor of colliding particles (temperature dependent) Potential Energy Potential Energy transition state Eact Separate Reactants products of reaction DH Progress of Reaction Distribution of Energy dN dE Fraction is exp( Energy -Eact RT ) Arrhenius Equation Rr = ( X )(e Eact RT Rr = ( A)(e )( Z ) Eact RT ) E act ln Rr = ln A RT (9) (10) (11) Arrhenius Plot A ln Rr Eact R 1 high temperature (lab tests) low temperature (site temperature) Inverse Temperature (1/T) Experimental Design • Incubation environment should simulate the field (i.e., landfill environment) – Limited Oxygen – Some degree of liquid extraction • Utilize elevated temperatures to accelerate the reactions. – 55, 65, 75, and 85oC Incubation Device 1 10 Piezometer Load Insulation Perforated steel loading plate Sand Sand Heat tape Geomembrane Tests Performed • Oxidative inductive time (OIT) for antioxidant content. • Melt index for qualitative molecular weight measurement. • Tensile test for mechanical property OIT Tests • OIT is the time required for the polymer to be oxidized under a specific test condition. • OIT value indicates the total amount (not the type) of the antioxidant remaining in the polymer. OIT Test for Evaluation of Antioxidant (AO) • OIT Tests: – ASTM D3895-Standard OIT (Std-OIT), or – ASTM D 5885-High Pressure OIT (HP-OIT) • HP-OIT test is used for AOs which are sensitive to high temperature testing Thermal Curve of OIT Test Test Results Percent Retained 150 Std-OIT HP-OIT Density Melt Index Yield Stress Yield Strain Break Stress Break Strain 100 50 0 0 5 10 15 20 25 30 Incubation Time (month) Changes in Eight Properties with Incubation Time at 85°C Analysis of OIT Data a. Determine OIT depletion rate at each temperature. b. Utilize Arrhenius Equation to extrapolate the depletion rate to a lower temperature. c. Predict the time to consume all antioxidant in the polymer. a) - OIT Depletion Rate 4.5 ln OIT (min.) 4 3.5 3 2.5 55°C 65°C 75°C 85°C 2 1.5 1 0 5 10 15 Incubation Time (month) 20 25 b) –Arrhenius Plot -1 ln (OIT Depletion Rate) Standard OIT y = 17.045 - 6798.2x R^2 = 0.953 HP-OIT y = 16.856 - 6991.3x R^2 = 0.943 -2 -3 -4 -5 0.0027 0.0028 0.0029 1/T (°K) 0.0030 0.0031 c) Lifetime of Antioxidant • Use the OIT depletion equation to find “t” ln(OIT) = ln(P) – (S) * (t) • The OIT value for unstabilized PE is 0.5 min. • For this particular stabilization package t = 200 years Lifetime of Geomembrane • Induction time and degradation period (Stages B & C) can be established by using unstabilized polymer in the experiment. • It was found by Gedde et al. (1994) that the duration of Stages B and C is significant shorter than that of Stage A. • Antioxidants are critical to the long-term performance of polyethylene and other polyolefines. Future of Waste Containment • Current waste containment technique is defined as “dry dome” method by eliminating leachate from being generated after closure. • Waste will not degrade since moisture is a critical component of the biodegradation process. Bioreactor Landfill “……a sanitary landfill operated for the purpose of transforming and stabilizing the readily and moderately decomposable organic waste constituents within five to ten years following closure by purposeful control to enhance microbiological processes. The bioreactor landfill significantly increases the extent of waste decomposition, conversion rates and process effectiveness over what would otherwise occur within the landfill.” Why Operate a Landfill as a Bioreactor? • to increase potential for waste to energy conversion, • to store and/or treat leachate, • to recover air space, and • to ensure sustainability Status • 1993 - less than 20 landfills recirculating leachate • 1997 - ~ 130 landfills recirculating leachate • My estimate - ~ 5% of landfills Aerobic Bioreactor • • • • Rapid stabilization of waste Enhanced settlement Evaporation of moisture Degradation of organics which are recalcitrant under anaerobic conditions • Reduction of methane emissions Research Issues - Aerobic Bioreactor • • • • • How much air is needed? How can air be delivered? What is the impact on the water balance? How are landfill fires prevented? What are the economic implications?