Colon Cancer Screening Ryan Burris James Frye Melvie Kim
advertisement

Colon Cancer Screening
Ryan Burris
James Frye
Melvie Kim
Nicholas Lee
Jennifer Mah
Hoa Nguyen
Epidemiology
• Colorectal cancer is the third most common
cancer in the US (70% arise in the colon)
• Each year there are approximately 133,000 new
cases diagnosed and approximately 50,000
deaths (8% of all cancer deaths)
• Worldwide highest incidence in Australia, New
Zealand, Europe and North America. Lowest
incidence in Africa and South Central Asia
• Incidence in US has declined 2-3% per year over
the last 15 years (increased screening and polyp
removal)
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
Risk Factors
Age (incidence increases between 40-50 yo)
Men (incidence 25% higher )
African American ( incidence 20% higher than whites)
Family History (first degree relatives, age of onset)
Hereditary CRC Syndromes (FAP, HNPCC-5% of call CRC cases)
Personal History of Large (>1 cm) adenomatous polyps w/ villous or
tubulovillous histology or high grade dysplasia
Inflammatory Bowel Disease (UC, fewer data to suggest Crohn’s disease)
Abdominal Radiation
Acromegaly
Diabetes (? Insulin is growth factor for colonic mucosal cells)
Renal Transplant on Immunosuppression
Low Socioeconomic Status
Unhealthy Diet (red and processed meats {BACON}, high temperature
cooking)
Smoking and Alcohol
Obesity
Protective Factors
•
•
•
•
Regular Physical Activity
Fruits and Vegetables
Fiber
ASA and NSAIDs (?increased apoptosis and
impairment of tumor cell growth by inhibition of
COX-2)
• Post Menopausal Hormonal Therapy
• ?Folic Acid, Folate, Vitamin B6,
Dietary/Supplemental Calcium, Vitamin D,
Magnesium, Garlic, Omega 3 Fatty Acids, Statins
Pathogenesis
• Involves a sequence of events resulting in the
transformation of adenomatous polyps to cancer
• Early mutations in adenomatous polyposis coli
gene (APC) lead to initial hyperproliferative state
• Accumulation of additional mutations and
abnormal DNA methylation further drives the
malignant transformation from adenoma to
carcinoma
Pathogenesis
• CRC spreads through the lymphatics,
hematogenously or by contiguous and
transperitoneal routes.
• The TNM staging system is used for CRC
• Metastasis is often to regional lymph nodes,
liver, lungs and peritoneum
Familial Adenomatous Polyposis
• Characterized by numerous colonic adenomas
appearing during childhood with significant
malignant potential (90% will develop cancer
by 45 yo)
• Symptoms occur at approximately 16 yo
• Secondary to germline mutations in the
adenomatous polyposis coli (APC) gene on
chromosome 5
Hereditary Non-Polyposis Colorectal
Cancer
•
•
•
•
Also known as Lynch Syndrome
More common than FAP
Autosomal Dominant
Caused by defects in the mismatch repair genes
(hMLH1, hMSH2, hMSH6, PMS2)
• Characterized by early age of onset and right
sided tumors (70% proximal to splenic flexure)
• Extracolonic cancers common (endometrial,
ovary, stomach, small bowel, hepatobiliary, brain,
renal, breast, prostate)
Clinical Presentation
• Patients often present in three ways
– Suspicious symptoms and/or signs
– Asymptomatic individuals discovered by routine
screening (over 30% of all CRCs)
– Emergency admission with intestinal obstruction,
peritonitis, or rarely an acute GI bleed
• Most are diagnosed after symptom onset
Clinical Presentation
• Signs and Symptoms
–
–
–
–
–
–
–
–
–
Hematochezia (more commonly left sided tumors)
Melena (more commonly right sided tumors)
Iron Deficiency Anemia (more commonly right sided tumors)
Abdominal Pain (tumors at all sites due to obstruction, peritoneal spread,
perforation)
Change in Bowel Habits (more commonly left sided tumors)
Nausea/Vomiting (obstructive signs)
Abdominal Distention
Tenesmus (Rectal CA)
Rectal Pain (Rectal CA)
• Atypical Presentations
–
–
–
–
Malignant fistula formation (bladder, small bowel)
Fever of Unknown Origin
Abscesses
Streptococcus bovis bacteremia
Screening Modalities
• Stool-Based Test
Detection of hemoglobin in stool
– Guaiac-based Fecal Occult Blood Test (gFOBT)
(chemical guaiac)
– Sensitive FOBT (chemical guaiac)
– Fecal Immunochemical Test (antibodies)
Limitations of Stool-Based Test: Not good for polyp
detection and many false-positives
Advantages: Low cost, risk and complexity of performing
test
Screening Modalities
• Colon Imaging and Direct Visualization
– Double-contrast barium enema
• Advantages: Relatively safe and can view entire colon
• Limitations: Abnormal results still need colonoscopy, falsepositives from retained stool, air, and other mucosal
irregularities
– Sigmoidoscopy
• Advantages: Bowel prep less onerous than for colonoscopy
and can be performed without sedation
• Limitations: Screens up to splenic flexure only, can cause
perforation, abnormal tests requires follow up colonoscopy
Screening Modalities
– CT Colonography
• Advantages: No sedation required
• Limitations: Aggressive bowel prep required, abnormal
results require follow up colonoscopy
– Capsule Endoscopy
• Advantages: Less invasive
• Limitations: Not an option for initial test, only for
incomplete colonoscopy. Requires more rigorous bowel
prep, no intervention thus requires follow up
colonoscopy for abnormal results
Screening Modalities
– Colonoscopy
**Preferred option by American College of
Gastroenterology
• Advantages: intervention can be completed during
same procedure, can detect proximal lesions better
than sigmoidoscopy
• Limitations: risk of perforation or bleeding (1 in 1000),
expensive, conscious sedation, bowel prep
Screening Modalities
• Investigational
– Chromoendoscopy, magnification endoscopy,
narrow band imaging optical colonoscopy
– Serum markers
• Septin 9 hypermethylation
• Seven gene test
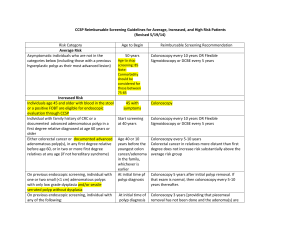
SCREENING
Two major United States guidelines were released in 2008
1) Multi-Society Task Force
2) the US Preventive Services Task Force (USPSTF)
Two major United States guidelines were released in 2008
Multi-Society Task Force
-prefers tests that can prevent cancer over those that mainly offer early
detection of cancers.
-more inclusive in the tests it recommended
(the best test is the one patients will take)
the US Preventive Services Task Force
Two major United States guidelines were released in 2008
Multi-Society Task Force
-prefers tests that can prevent cancer over those that mainly offer early
detection of cancers.
-more inclusive in the tests it recommended
(the best test is the one patients will take)
the US Preventive Services Task Force
-required a higher level of evidence for including a test.
-more explicit about the age to stop screening.
Whether to recommend screening >70 years of age should depend upon the health
status, anticipated life expectancy, risk for colorectal cancer, and personal values
Whether to recommend screening >70 years of age should depend upon the health
status, anticipated life expectancy, risk for colorectal cancer, and personal values
Patients with a life expectancy less than ten years (some would say five)
would not
be expected to benefit from colorectal screening, since studies
indicate benefit
from screening starts to accrue after about five years.
Whether to recommend screening >70 years of age should depend upon the health
status, anticipated life expectancy, risk for colorectal cancer, and personal values
Patients with a life expectancy less than ten years (some would say five)
would not
be expected to benefit from colorectal screening, since studies
indicate benefit
from screening starts to accrue after about five years.
Colonoscopy carries increased risk in older adults, with significant
complications
occurring in 0.3 percent of 600 veterans aged 70 to 75 undergoing
colonoscopy
screening
There are no randomized, controlled trials of screening in people with a family history of
colorectal cancer. Screening recommendations are mostly based on expert opinion
The American College of Gastroenterology (ACG) issued guidelines in 2008
There are no randomized, controlled trials of screening in people with a family history of
colorectal cancer. Screening recommendations are mostly based on expert opinion
The American College of Gastroenterology (ACG) issued guidelines in 2008
Screen with colonoscopy.
There are no randomized, controlled trials of screening in people with a family history of
colorectal cancer. Screening recommendations are mostly based on expert opinion
The American College of Gastroenterology (ACG) issued guidelines in 2008
Screen with colonoscopy.
If a single first-degree relative was diagnosed at age 60 years or older with CRC
or an
advanced adenoma (≥1 cm, or high-grade dysplasia, or villous elements),
screening
with colonoscopy is recommended every 10 years beginning at age 50,
consistent
with one option for average risk screening
There are no randomized, controlled trials of screening in people with a family history of
colorectal cancer. Screening recommendations are mostly based on expert opinion
The American College of Gastroenterology (ACG) issued guidelines in 2008
Screen with colonoscopy.
If a single first-degree relative was diagnosed at age 60 years or older with CRC
or an
advanced adenoma (≥1 cm, or high-grade dysplasia, or villous elements),
screening
with colonoscopy is recommended every 10 years beginning at age 50
or
age
If a single first-degree relative was diagnosed before 60 years with CRC or an
advanced adenoma, or two or more first-degree relatives had colorectal cancer
advanced adenomas at any age, screening with colonoscopy is recommended at
40 or 10 years before the youngest relative's diagnosis, to be repeated every five
years.
Classic FAP
If genetic testing cannot be done or is uninformative:
Screening gene carriers or at-risk family members with flexible sigmoidoscopy or
colonoscopy every 12 months starting around age 10 to 12 years and continuing until age
35 to 40 years if negative.
Once colonic polyposis is established, a full colonoscopy should be performed to
evaluate the extent of the colonic polyposis.
Colectomy near the time of initial diagnosis in patients with profuse polyposis, multiple
large (>1 cm) adenomas, or adenomas with villous histology and/or high-grade dysplasia.
Patients with sparse, small (<5 mm) adenomas can usually be followed endoscopically
with surgery scheduled to accommodate school and work schedules.
Patients who have undergone total proctocolectomy require regular surveillance of the
ileal pouch.
Lynch syndrome
CRC screening with colonoscopy
-every 1to 2years beginning at age 20-25 years,
-or two to five years prior to the earliest age of CRC diagnosis in the family
Lynch syndrome
CRC screening with colonoscopy
-every 1to 2years beginning at age 20-25 years,
-or two to five years prior to the earliest age of CRC diagnosis in the family
(whichever comes first)
Surveillance
Surveillance
Low-risk adenomas – If only one or two small (≤10 mm) tubular adenomas are found on
baseline colonoscopy, the first surveillance colonoscopy should be performed in 5 to 10
years
Surveillance
Low-risk adenomas – If only one or two small (≤10 mm) tubular adenomas are found on
baseline colonoscopy, the first surveillance colonoscopy should be performed in 5 to 10
years
Individuals with advanced adenomas (≥10 mm, villous histology or high-grade dysplasia)
or between 3 and 10 adenomas on their first surveillance colonoscopy should undergo
their next surveillance colonoscopy in three years
Surveillance
Low-risk adenomas – If only one or two small (≤10 mm) tubular adenomas are found on
baseline colonoscopy, the first surveillance colonoscopy should be performed in 5 to 10
years
Individuals with advanced adenomas (≥10 mm, villous histology or high-grade dysplasia)
or between 3 and 10 adenomas on their first surveillance colonoscopy should undergo
their next surveillance colonoscopy in three years
patients with more than 10 adenomas should be evaluated for a hereditary colorectal
cancer syndrome and have surveillance colonoscopy in less than three years
Colorectal cancer surveillance in IBD
•
•
•
•
Necessary.
Lack of evidence on the interval and location
Grade 1B/Strong recs: colectomy for high grade dysplasia
Grade 2B/Weak recs:
• colectomy for those with low-grade dysplasia
• UC past splenic flexure or Crohn colitis, first colonoscopy 8
yrs after disease onset then annually
• Grade 2C/Very weak recs:
– left-sided UC, begin colonoscopy after 12 years of disease; then
annually
CRC MKSAP
Question 1
A 57-year-old woman is evaluated after a recent
screening colonoscopy. The colonoscopy
disclosed a 12-mm polyp in the ascending colon,
which was removed. No other lesions were
noted. On pathology, the lesion was found to be
a sessile serrated polyp.
Physical examination findings are unremarkable.
Which of the following is the most appropriate
time to repeat colonoscopy?
A. 1 year
B. 3 years
C. 5 years
D. 10 years
B. 3 years
• Hyperplastic polyps
– No malignant potential
• Sessile serrated polyps
– Precursor of 15% of sporadic colorectal cancers
– <10 mm repeat colonoscopy in 5 years
– ≥ 10mm repeat colonoscopy in 3 years
• Serrated adenomas
Key Point
For patients with large (≥10mm) or dysplastic
sessile serrated polyps or traditional serrated
adenomas, the recommended
postpolypectomy surveillance colonoscopy
interval is 3 years.
Question 2
A 65-year-old man is evaluated after a recent
colonoscopy, which disclosed a 2.5-cm pedunculated
polyp in the sigmoid colon. The polyp was removed in
its entirety in one piece. Biopsy results showed a welldifferentiated adenocarcinoma confined to the
submucosa without evidence of lymphovascular
involvement and a 1-mm margin. There is no family
history of colorectal cancer.
Physical examination findings are unremarkable.
Which of the following is the most appropriate
management?
A. Colon resection
B. CT scan of the abdomen and pelvis
C. Radiation therapy
D. Repeat colonoscopy in 3 months
D. Repeat colonoscopy in 3 months
• Invasive adenocarcinoma in a pedunculated
polyp is adequately treated by endoscopic en
bloc polypectomy alone if:
– Confined to the submucosa
– Not poorly differeniated
– No lymphatic or vascular invasion
– No involved margins
D. Repeat colonoscopy in 3 months
• If adverse histologic features are noted, there
is increased risk of lymph node involvement
and surgical resection is required
• Consider surgery if the lesion is removed
piecemeal
Key Point
National recommendations for postpolypectomy
surveillance intervals are as short as 3 to 6
months in patients with large (>2cm)
adenomas with invasive cancer and favorable
prognostic features.
Question 3
A 62-year-old man is evaluated after a recent
screening colonoscopy. The colonoscopy disclosed a
3-mm sigmoid polyp and an 8-mm hepatic flexure
polyp, both of which were removed. On pathology,
the sigmoid polyp is noted to be a hyperplastic
polyp, and the hepatic flexure polyp is found to be a
tubulovillous adenoma with high-grade dysplasia.
Physical examination findings are unremarkable.
Which of the following is the most appropriate
time to repeat colonoscopy?
A. 3 to 6 months
B. 1 year
C. 3 years
D. 5 years
C. 3 years
• Adenomas = increased risk for colon cancer
• Low risk next colonoscopy in 5 years
– 1 or 2 adenomas smaller than 10mm
• High risk next colonoscopy in 3 years
– Adenoma 10mm or larger
– 3 to 10 adenomas
– Adenoma with villous component (tubulovillous
or villous adenoma)
– Adenoma with high-grade dysplasia
Question 4
A 72-year-old man is evaluated during a routine
examination. He underwent a sigmoid
colectomy and adjuvant chemotherapy 4 years
ago for stage III adenocarcinoma of the colon.
Results of the colonoscopies performed 1 year
postoperatively and last year were normal.
On physical examination, he appears healthy
and has no evidence of recurrent disease.
Which of the following is the most appropriate
time to repeat colonoscopy?
A. 1 year
B. 3 years
C. 5 years
D. 10 years
C. 5 years
• Screening recommendations for patients with
a history of colorectal cancer consists of
follow-up colonoscopy at 1 year and 3 years
after curative surgical resection
• If results of these colonoscopies are normal,
the surveillance interval can be extended to 5
years
Question 5
A 38-year-old woman is evaluated in follow-up
after recent surgery for endometrial cancer. Her
family history is significant for colon cancer in
her sister (diagnosed at age 45 years) and her
mother (diagnosed at age 65 years). Her
maternal grandfather was diagnosed with rectal
cancer at age 47 years. The patient has never
had a colon cancer screening with colonoscopy.
Which of the following is the most appropriate
time to start colon cancer screening with
colonoscopy?
A. Now
B. Age 40 years
C. Age 47 years
D. Age 50 years
A. Now
• Hereditary nonpolyposis colorectal cancer
• Amsterdam II criteria
– 3 or more relatives with HNPCC-associated cancer
• Colorectal, endometrial, ovarian, urothelial, gastric,
brain, small bowel, hepatobiliary, skin
– 2 successive generations of relatives affected
– 1 affected relative a first-degree relative or 2 other
affected relatives
– 1 cancer diagnosed before age 50 years
A. Now
• Surveillance colonoscopy is indicated for
patients who
– Meet the clinical criteria for HNPCC
– Have Lynch syndrome (presence of germline
genetic mutation)
– Are at risk for Lynch syndrome and have not had
genetic testing
A. Now
• The recommended surveillance interval for
colonoscopy screening in patients who have
or at risk for Lynch syndrome is
– Every 1 to 2 years beginning at age 25 years, or
– 2 to 5 years earlier than the youngest age at
diagnosis of colorectal cancer if the affected
relative was younger than 25 years old
References
• UpToDate
• Medscape