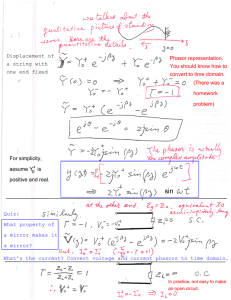
i INVESTIGATION ON QUALITY OF HYDROXYAPATITE ADHESION ON INVESTMENT CASTING MOULD
advertisement

i INVESTIGATION ON QUALITY OF HYDROXYAPATITE ADHESION ON INVESTMENT CASTING MOULD AMIR FEREIDOUNI LOTFABADI A thesis submitted in fulfillment of the requirements for the award of the degree of Master of Engineering (Mechanical Engineering) Faculty of Mechanical Engineering Universiti Teknologi Malaysia JULY 2008 iii To my beloved father and mother Amir 28/7/2008 iv ACKNOWLEDGEMENT In this research, I was in contact with many people, researchers, academicians, and practitioners. They have contributed towards my understanding and thoughts. In particular, I wish to express my sincere appreciation to my main thesis supervisor, Associate Professor Dr. Hasbullah Haji Idris, for support, guidance, critics and friendship. I am also very thankful to my co-supervisors Dr. Mohd Rafiq. Foundry lab technicians in Universiti Teknologi Malaysia had very kind and helpful cooperation with me during my research as well. Furthermore my Friends Mr. Hessam Majd and Mr. Ali Akhavan Farid had very helpful guidance and sympathy during my research time. v ABSTRACT Quality of Hydroxyapatite adhesion on investment casting mould was investigated in this project. Investment casting is a new method for HAp coating onto the metals. First stage of applying this method is making appropriate investment casting mould. Appropriate investment casting mould should have specific properties such as: sufficient strength, proper shape for obtaining sound casting, and the must important one, enough amount of HAp should adhered onto the inner layer of investment casting mould to defuse into the metal during casting for desirable coating. For this purpose appropriate methods used to stick sufficient amount of Hydroxyapatite onto inner layer of ceramic investment casting mould to prepare it for metal coating by casting. therefore 3 different HAp-water mixture viscosities: 5, 7.5 and 10 seconds, were applied to find out which of them was support enough amount of Hydroxyapatite after dewaxing and firing. Dewaxing in three different temperatures 100°, 200° and 300° C applied as well to investigate the effect of the dewaxing temperature on the quality of HAp adhesion on to the moulds. Finally after gathering the results of dewaxing; moulds that have the desirable properties were fired at 600° C to study the effect of firing process on the quality of hydroxyapatite adhesion on moulds. After all XRD, EDAX tests and 3D microscope supervision were done to find out the results. By considering these tests 5 seconds viscosity of HAp-water mixture and 300°C dewaxing temperature had the desirable properties for making sufficient investment casting moulds for metal coating. vi TABLE OF CONTENTS CHAPTER TITLE PAGE DECLARATION ii DEDICATION iii ACKNOWLEDGMENT iv ABSTERACT v TABLE OF CONTENTS vi LIST OF TABLES ix LIST OF FIGUERES x,xi 1 INTRODUCTION 1 2 TISSUE ENGINEERING 4 2.1 Introduction 4 2.2 Bio-active materials for tissue engineering 6 2.2.1 Ceramics 8 2.2.1.1 Nonabsorbable or Relatively Bioinert Bioceramics 10 2.2.1.2 Biodegradable or Resorbable Ceramics 11 2.2.1.3 Bioactive or Surface-Reactive Ceramics 12 2.2.1.4 Bioceramics Calcium Phosphate base 13 2.2 Hydroxyapatite 14 2.2.1 Identification 15 2.2.2 Advantage of Hydroxyapatiate 15 2.2.3 Disadvantage of Hydroxyapatite 16 vii CHAPTER 3 4 TITLE PAGE 2.2.4 Hydroxyapatite coatings 16 2.2.5 Coating Techniques 16 2.3 Related works on usage of Hydroxyapatite 18 2.3.1 Bioactive coatings on 316L Stainless Steel implants 18 2.3.2 Hydroxyapatite coating on cobalt base alloys 19 METHODOLOGY AND EXPERIMENTAL PROCEDURE 23 3.1 Introduction 23 3.2 Methodology and Experiment 24 3.2.1 Design 26 3.2.1.1 Pattern Design 26 3.2.1.2 Design of wax mould 27 3.2.2 Mould Fabrication 30 3.2.3 Wax pattern manufacture 31 3.2.4 Ceramic mould fabrication 33 3.2.4.1 Preparing wax samples 34 3.2.4.2 Preparing Hydroxyapatite for coating 34 3.2.4.3 Coating of samples 35 3.2.4.4 Slurry preparation 37 3.2.4.5 Investment casting mould making 39 3.2.5 Effect of dewaxing temperature 42 3.2.5.1 Introduction 42 3.2.5.2 Dewaxing 42 3.2.6 Firing process 44 3.2.7 Tests 44 RESULTS AND DISCCUSION 48 4.1 XRD Result 48 4.2 EDAX Result 59 4.3 3D microscope 63 viii CHAPTER 5 TITLE PAGE COCLUSTION 64 REFRENCES 66 ix LIST OF TABLES TABLE NO. TITLE PAGE 2.1 Coating techniques of Hydroxyapatite 16-17 2.2 Summarized related works on HAp Coatings 20-22 4.1 XRD test results – Reference Materials 52 4.2 XRD test results for different viscosities 57 4.3 EDAX result for 600°C temperature 60 4.4 EDAX result for actual samples, Fluka 62 x LIST OF FIGUERS FIGURE NO. TITLE PAGE 2.1 Hydroxyapatite structure projected 14 3.1 Flowchart of the process 25 3.2 Drawing of a sample 27 3.3 Base part of pattern mould 28 3.4 Middle part of the pattern mould 29 3.5 Top part of the pattern mould 29 3.6 Disassembled pattern mould 31 3.7 Assembled mould for wax pattern 31 3.8 Solid wax pattern inside the mould 33 3.9 Wax patterns hanged for drying 33 3.10 Mixing HAp and mineral water 35 3.11 Measuring the viscosity of HAp-water mixture 36 3.12 Preparing ceramic slurry 38 3.13 Coating wax patterns with ceramic slurry 40 3.14 Coated wax pattern with slurry 40 3.15 Moulds after stuccoing 41 3.16 Moulds after final coating 41 3.17 Making holes inside the wax 43 3.18 Moulds inside the furnace 43 3.19 Broken samples parts for tests 47 3.20 Prepared powder for XRD test 47 xi FIGURE NO. TITLE PAGE 4.1 XRD result for Fluka 49 4.2 XRD result for Granumas 50 4.3 XRD result for Iran 51 4.4 XRD result for reference materials 53 4.5 XRD result for 5S viscosity and 600°C 54 4.6 XRD result for 7.5 viscosity and 600° C 55 4.7 XRD result for 10 viscosity and 600° C 56 4.8 Material amount vs. Temperature in 5S viscosity 58 4.9 Material amount vs. Temperature in 7.5S viscosity 58 4.10 Material amount vs. Temperature in 10S viscosity 59 4.11 Adhesion of HAp onto the inner layer of mould 64 CHAPTER 1 INTRODUCTION Hydroxylapatite, also called hydroxyapatite, is a mineral. It is a naturally occurring form of calcium apatite with the formula Ca5 (PO4)3 (OH), but is usually written Ca10 (PO4)6(OH)2 to denote that the crystal unit cell comprises two molecules. Hydroxylapatite is the hydroxyl endmember of the complex apatite group. The OHion can be replaced by fluoride, chloride or carbonate. It crystallizes in the hexagonal crystal system. It has a specific gravity of 3.08 and is 5 on the Mohs hardness scale. Pure hydroxylapatite powder is white. Naturally occurring apatites can however also have brown, yellow or green colorations.. Hydroxylapatite can be found in teeth and bones, within the human body. Therefore, it can be used as a filler to replace amputated bone or as a coating to promote bone ingrowth into prosthetic implants. Although many other phases exist with similar or even identical chemical makeup, the body responds much differently to them. Many modern implants, e.g hip replacements and dental implants, are coated with hydroxyapatite. It has been suggested that this may promote osseointegration and there is good evidence for this. Because of its poor mechanical strength must of 2 the time it is needed to use it as a coating of any other materials such as Titanium alloys, Cobalt alloys and medical grade Stainless steel. There are various ways for coating HAp on to different materials each method of coating has its own advantage and disadvantages; The method of coating is dipend on the usage of the implant, accuracy, amount of HAp that was needed to coat and the cost of the method as well. Some of these methods are summarized in table 2.1. in chapter 2. One of the most new methods for coating HAp on to the metallic materials is coating by investment casting method, also called lost-wax casting, is one of the oldest known metal-forming techniques. From 5,000 years ago, when beeswax formed the pattern, to today’s high-technology waxes, refractory materials and specialist alloys, the castings allow the production of components with accuracy, repeatability, versatility and integrity in a variety of metals and high-performance alloys. Lost foam casting is a modern form of investment casting that eliminates certain steps in the process. Investment casting consist of 3 main stage, at first making the wax and preparing ceramic shell on it, second dewaxing the moulds and finally fire the dewaxed moulds. This method of coating firstly used to coat HAp on to Cobalt alloys [J.C. Escobedo, et. al. 2006]. This method of coating has its own advantages and disadvantages. This method is very cheap regarding to other coating methods and it is very simple as well. Also according to using simple methods control of the elements which are involved in the finale results is easier than other coating methods like plasma spraying or electro chemical methods for coating. In this project finding the best dewaxing temperature for moulds aimed. And investigation to find out what is the best viscosity of HAp-water mixture for coating the inner layer of investment casting moulds was targeted as well. 3 This report started from introduction on tissue engineering and highlight its history and importance through human life also mentioned the materials and methods that used and being in the process from past until now. Afterward the properties, composition and classification of bioceramics discussed in detail especially for hydroxyapatite. Also the related works, investigations and applying of several bioceramics issued in several tables. At 3rd chapter methodology and experimental procedure discussed, 4th and 5th chapter talked about on results and conclusion as well. 4 CHAPTER2 TISSUE ENGINEERING 2.1 Introduction Biomedical engineering (BME) is the application of engineering principles and techniques to the medical field. It combines the design and problem solving skills of engineering with the medical and biological science to help improve patient health care and the quality of life of healthy individuals. As a relatively new discipline, much of the work in biomedical engineering consists of research and development, covering an array of fields: bioinformatics, medical imaging, image processing, physiological signal processing, biomechanics, biomaterials and bioengineering, systems analysis, 3-D modeling, etc. Examples of concrete applications of biomedical engineering are the development and manufacture of biocompatible protease. Tissue Engineering is the use of a combination of cells, engineering and materials methods, and suitable biochemical and physio-chemical factors to improve or replace biological functions. While most definitions of tissue engineering cover a broad range of applications, in practice the term is closely associated with applications that repair or replace portions of or whole tissues (i.e., bone, cartilage, blood vessels, bladder, etc). Often, the tissues involved require certain mechanical and structural properties for proper function. The term has also been applied to efforts 5 to perform specific biochemical functions using cells within an artificially-created support system (e.g. an artificial pancreas, or a bioartificial liver). The term regenerative medicine is often used synonymously with tissue engineering, although those involved in regenerative medicine place more emphasis on the use of stem cells to produce tissues. Natural bone surface is quite often contains features that are about 100 nm across. If the surface of an artificial bone implant were left smooth, the body would try to reject it. Smooth surface is likely to cause production of fibrous tissue covering the surface of the implant. This layer reduces the bone-implant contact, which may result in loosening of the implant and further inflammation. It was demonstrated that by creating nano-sized features on the surface of the hip or knee prosthesis one could reduce the chances of rejection as well as to stimulate the production of osteoblasts. The osteoblasts are the cells responsible for the growth of the bone matrix and are found on the advancing surface of the developing bone.The effect was demonstrated with polymeric, ceramic and, more recently, metal materials. More than 90% of the human bone cells from suspension adhered to the nanostructured metal surface [Gutwein, LG, et. al. 2003], but only 50% in the control sample. In the end this findings would allow to design a more durable and longer lasting hip or knee replacements and to reduce the chances of the implant getting loose. Titanium is a well-known bone repairing material widely used in orthopaedics and dentistry. It has a high fracture resistance, ductility and weight to strength ratio. Unfortunately, it suffers from the lack of bioactivity, as it does not support sell adhesion and growth well. Apatite coatings are known to be bioactive and to bond to the bone. Hence, several techniques were used in the past to produce an apatite coating on titanium. Those coatings suffer from thickness non-uniformity, poor adhesion and low mechanical strength. In addition, a stable porous structure is required to support the nutrients transport through the cell growth. It was shown that using a biomimetic approach – a slow growth of nanostructured apatite film from the simulated body fluid – resulted in the formation 6 of a strongly adherent, uniform nanoporous layer [Ma J, et. al. 2003]. The layer was found to be built of 60 nm crystallites, and possess a stable nanoporous structure and bioactivity. A real bone is a nanocomposite material, composed of hydroxyapatite crystallites in the organic matrix, which is mainly composed of collagen. The bone is mechanically tough and, at the same time, plastic, so it can recover from a mechanical damage. The actual nanoscale mechanism leading to this useful combination of properties is still debated. 2.1 Bio-active materials for tissue engineering: The first generation of biomedical materials used within the body was largely biologically inert, or nearly-inert. The goal at the time was to achieve a suitable combination of physical properties to match those of the replaced tissue with a minimal toxic response in the host. It was once thought that all materials, when placed inside the body, would elicit a foreign body response, the formation of a nonadherent fibrous capsule around the implant. However the discovery, in 1969, of a four-component glass which could bond to living tissue showed that it is possible for certain materials to elicit a controlled action and reaction in the physiological environment. By the mid-1980s bioactive materials had reached clinical use in a variety of orthopaedic and dental applications, including various compositions of bioactive glasses, ceramics, glass-ceramics and composites. Another advance in this second generation of materials for medicine was the development of resorbable biomaterials, designed to break down chemically and be resorbed at an equivalent rate to tissue regrowth. Ultimately the foreign material is replaced by regenerating tissue and the implant site becomes virtually indistinguishable from the host tissue. An example of this is the biodegradable suture, in which the polymer composed of polylactic (PLA) and polyglycolic (PGA) acids decomposes and metabolises into CO2 and H2O. 7 Developments throughout the last century, such as drugs, vaccines, water treatment and improved hygiene have resulted in a vast increase in the average life expectancy in developed countries. While the clinical success of bioinert, bioactive and resorbable implants has greatly improved the quality of life for tens of millions of people, research shows that a third to half of prostheses fail within 10–25 years and patients require revision surgery. Twenty years of research have had only small effects on failure rates. In fact the improvement of first- and second-generation materials is limited as all man-made biomaterials used for the repair or restoration of the body represents a compromise. Synthetic materials cannot respond to changing physiological loads or biochemical stimuli, unlike living tissues. This limits the lifetime of artificial body parts. Thus there is a need to consider a shift towards a more biologically based method for the repair and regeneration of tissues. The new challenge in biomaterials is to enhance the body’s own regenerative capacity by stimulating genes which initiate repair at the site of damage or disease. A third generation of biomaterials is being developed to do this. The separate concepts of bioactive and resorbable materials have been combined to make bioactive materials resorbable. Third generation bioactive glasses and macroporous foams are being designed to activate genes that stimulate regeneration of living tissues. Molecular modifications of resorbable polymer systems elicit specific cellular responses. Tissue-engineered constructs can be produced by seeding progenitor cells onto modified resorbable scaffolds. The cells grow outside the body, become differentiated and mimic naturally occurring tissues. The construct can then be implanted into a patient. In time the scaffold is resorbed and replaced by host tissue that includes a viable blood supply and nerves. The living construct will adapt to the physiological environment and should provide long-lasting repair. Biomaterials can be used in situ in the form of powders, solutions or doped microparticles to stimulate local tissue repair. The materials release chemicals in the 8 form of ionic dissolution products, or growth factors such as bone morphogenic protein (BMP), at controlled rates, by diffusion or network breakdown, which activates the cells in contact with the stimuli. The cells produce additional growth factors that in turn stimulate multiple generations of growing cells to self-assemble into the required tissues in situ. For example, when a particulate bioactive glass is used to fill a bone defect there is rapid regeneration of bone that matches the architecture and mechanical properties of bone at the site of repair. [V.J shirtliff,LL Hench 2003]. 2.1.1 Ceramics Ceramics are defined as the art and science of making and using solid articles that have as their essential component, inorganic nonmetallic materials [Kingery et al., 1976]. Ceramics are refractory, polycrystalline compounds, usually inorganic, including silicates, metallic oxides, carbides and various refractory hydrides, sulfides, and selenides. Oxides such as Al2O3, MgO, SiO2, and ZrO2 contain metallic and nonmetallic elements and ionic salts, such as NaCl, CsCl, and ZnS [Park and Lakes, 1992]. Exceptions to the preceding include covalently bonded ceramics such as diamond and carbonaceous structures like graphite and pyrolized carbons [Park and Lakes, 1992]. Ceramics in the form of pottery have been used by humans for thousands of years. Until recently, their use was somewhat limited because of their inherent brittleness, susceptibility to notches or micro cracks, low tensile strength, and low impact strength. However, within the last 100 years, innovative techniques for fabricating ceramics have led to their use as “high tech” materials. In recent years, humans have realized that ceramics and their composites can also be used to augment or replace various parts of the body, particularly bone. Thus, the ceramics used for the latter purposes are classified as bioceramics. Their relative inertness to the body fluids, high compressive strength, and aesthetically pleasing appearance led to the use of ceramics in dentistry as dental crowns. Some carbons have found use as implants 9 especially for blood interfacing applications such as heart valves. Due to their high specific strength as fibers and their biocompatibility, ceramics are also being used as reinforcing components of composite implant materials and for tensile loading applications such as artificial tendon and ligaments [Park and Lakes, 1992]. Unlike metals and polymers, ceramics are difficult to shear plastically due to the (ionic) nature of the bonding and minimum number of slip systems. These characteristics make the ceramics non-ductile and are responsible for almost zero creep at room temperature [Park and Lakes, 1992]. Consequently, ceramics are very susceptible to notches or microcracks because instead of undergoing plastic deformation (or yield) they will fracture elastically on initiation of a crack. At the crack tip the stress could be many times higher than the stress in the material away from the tip, resulting in a stress concentration which weakens the material considerably. The latter makes it difficult to predict the tensile strength of the material (ceramic). This is also the reason ceramics have low tensile strength compared to compressive strength. If a ceramic is flawless, it is very strong even when subjected to tension. Flawless glass fibers have twice the tensile strengths of high strength steel (approximately 7 GPa) [Park and Lakes, 1992]. Ceramics are generally hard. Diamond is the hardest, with a hardness index of 10 on Moh’s scale, and talc (Mg3Si3O10COH) is the softest ceramic (Moh’s hardness 1), while ceramics such as alumina (Al2O3; hardness 9), quartz (SiO2; hardness 8), and apatite (Ca5P3O12F; hardness 5) are in the middle range. Other characteristics of ceramic materials are I. Their high melting temperatures II. Low conductivity of electricity and heat. These characteristics are due to the chemical bonding within ceramics. Ceramics used in fabricating implants can be classified as nonabsorbable (relatively inert), bioactive or surface reactive (semi-inert) [Hench, 1991, 1993] and biodegradable or resorbable (non inert) [Hentrich et al., 1971] and[Graves et al., 10 1972]. Alumina, zirconia, silicone nitrides, and carbons are inert bioceramics. Certain glass ceramics and dense hydroxyapatites are semi-inert (bioreactive) and calcium phosphates and calcium aluminates are resorbable ceramics [Park and Lakes, 1992]. Desired Properties of Implantable Bioceramics i. Should be nontoxic. ii. Should be no carcinogenic. iii. Should be no allergic. iv. Should be no inflammatory. v. Should be biocompatible. vi. Should be biofunctional for its lifetime in the host. Bioceramics are devided to 3 major classes i. Nonabsorbable or Relatively Bioinert Bioceramics, ii. The second class is Biodegradable or Resorbable Ceramics iii. Third is Bioactive or Surface-Reactive Ceramics. 2.1.1.1. Nonabsorbable or Relatively Bioinert Bioceramics: Relatively bioinert ceramics maintain their physical and mechanical properties while in the host. They resist corrosion and wear and have all the properties listed for bioceramics. Examples of relatively bioinert ceramics are dense and porous aluminum oxides, zirconia ceramics, and single phase calcium aluminates. (Relatively bioinert ceramics are typically used as structural-support implants. Some of these are bone plates, bone screws, and femoral heads. Examples of nonstructural support uses are ventilation tubes, sterilization devices [Feenstra and de Groot, 1983] and drug delivery devices. 11 Examples of Relatively Bioinert Bioceramics: i. Pyrolitic carbon coated devices ii. Dense and nonporous aluminum oxides iii. Porous aluminum oxides iv. Dense hydroxyapatites v. Zirconia ceramics Uses of Bioinert Bioceramics i. In the repair of the cardiovascular area. ii. As bone plates and screws. iii. In the form of ceramic-ceramic composites. iv. In the form of ceramic-polymer composites. v. As drug delivery devices. vi. As femoral heads. vii. As middle ear ossicles. viii. In the reconstruction of orbital rims. ix. As components of total and partial hips. x. In the form of sterilization tubes. xi. As ventilation tubes. xii. In reconstruction of acetabular cavities. 2.1.1.2. Biodegradable or Resorbable Ceramics Although Plaster of Paris was used in 1892 as a bone substitute [Peltier, 1961], the concept of using synthetic resorbable ceramics as bone substitutes was introduced in 1969 [Hentrich et. al., 1969] and [Graves et. al., 1972]. Resorbable ceramics, as the name implies, degrade upon implantation in the host. The resorbed material is replaced by endogenous tissues. The rate of degradation varies from 12 material to material. Almost all bioresorbable ceramics except Biocoral and Plaster of Paris (calcium sulfate dihydrate) are variations of calcium phosphate. Examples of resorbable ceramics are aluminum calcium phosphate, coralline, Plaster of Paris, hydroxyapatite, and tricalcium phosphate. Examples of Biodegradable Bioceramics: i. Glass Fibers and their composites. ii. Corals. iii. Calcium Sulfates, including Plaster of Paris iv. Zinc-Calcium-Phosphorous Oxides. v. Hydroxyapatites. vi. Tricalcium Phosphate. vii. Ferric Calcium Phosphorous Oxides viii. Aluminum-Calcium-Phosphorous Oxides. 2.1.1.3. Bioactive or Surface-Reactive Ceramics Upon implantation in the host, surface reactive ceramics form strong bonds with adjacent tissue. Examples of surface reactive ceramics are dense nonporous glasses, Bioglass and Ceravital, and hydroxyapatites (Table 3.5). One of their many uses is the coating of metal prostheses. This coating provides a stronger bonding to the adjacent tissues, which is very important for protheses. A list of the uses of surface-reactive ceramics is shown in (Table 3.5). Examples of Surface Reactive Bioceramics: i. For coating of metal prostheses ii. In reconstruction of dental defects. iii. For filling space vacated by bone screws, donor bone 13 iv. As bone plates and screws v. As replacements of middle ear ossicles. vi. In replacing subperiosteal teeth. vii. For correcting periodontal defects. viii. For lengthening of rami. 2.1.1.4. Bio ceramics calcium phosphate base The concept of using synthetic resorbable ceramics as bone substitutes was introduced in 1969 [Hentrich et al., 1969] , [Graveset al., 1972]. Resorbable ceramics , as the name implies, degrade upon implantation in the host. The resorbed material is replaced by endogenous tissues. The rate of degradation varies from material to material. Almost all bioresorbable ceramics except Biocoral and Plaster of Paris (calcium sulfate dihydrate) are variations of calcium phosphate. Calcium phosphate has been used in the form of artificial bone. This material has been synthesized and used for manufacturing various forms of implants, as well as for solid or porous coatings on other implants. Calcium phosphate can be crystallized into salts such as hydroxyapatite and β-whitlockite depending on the Ca:P ratio, presence of water, impurities, and temperature. In a wet environment and at lower temperatures (<900°C), it is more likely that hydroxyl- or hydroxyapatite will form, while in a dry atmosphere and at a higher temperature, β-whitlockite will be formed [Park and Lakes 1992]. Both forms are very tissue compatible and are used as bone substitutes in a granular form or a solid block. The apatite form of calcium phosphate is considered to be closely related to the mineral phase of bone and teeth. The mineral part of bone and teeth is made of a crystalline form of calcium phosphate similar to hydroxyapatite [Ca10 (PO4)6(OH)2]. The apatite family of mineral [A10 (BO4)6X2] crystallizes into hexagonal rhombic prisms and has unit cell dimensions α= 9.432 Å and c= 6.881 Å. The atomic structure of 14 . Note that the hydroxyl ions lie on the corners of the projected basal plane and they occur at equidistant intervals (3.44 Å) along the columns perpendicular to the basal plane and parallel to the c-axis. Six of the ten calcium ions in the unit cell are associated with the hydroxyls in these columns, resulting in strong interactions among them [Park and Lakes, 1992]. Figure 2.1: Hydroxyapatite structure projected down the c-axis onto the basal plane. 2.2. Hydroxyapatite Hydroxylapatite, also called hydroxyapatite, is a mineral. It is a naturally occurring form of calcium apatite with the formula Ca5 (PO4)3(OH), but is usually written Ca10 (PO4)6(OH)2 to denote that the crystal unit cell comprises two molecules. Hydroxylapatite is the hydroxyl endmember of the complex apatite group. The OHion can be replaced by fluoride, chloride or carbonate. It crystallizes in the hexagonal crystal system. It has a specific gravity of 3.08 and is 5 on the Mohs hardness scale. Pure hydroxylapatite powder is white. Naturally occurring apatites can however also have brown, yellow or green colorations. 70% of bone is made up of the inorganic mineral hydroxylapatite. Carbonated-calcium deficient hydroxylapatite is the main mineral of which dental enamel and dentin are comprised. Hydroxyapatite crystals are also found in the small 15 calcifications (within the pineal gland and other structures) known as corpora arenacea or 'brain sand'. 2.2.1. Identification: Molecular Weight: 502.31 gm Color: Colorless, White, Gray, Yellow, Yellowish green Crystal habit: Massive to crystalline, major bone forming mineral Crystal system: Hexagonal - Dipyramidal Cleavage: Indistinct Mohs scale hardness: 5 Luster: Vitreous to dull Refractive index: nω = 1.651 nε = 1.644 Optical Properties: Uniaxial (-) Birefringence: δ = 0.007 Streak: White Specific gravity: 3.08 Density: 3.156 g/cm^3 Diaphaneity: Transparent to Opaque 2.2.2. Advantages of Hydroxyapatite The beneficial biocompatible properties of hydroxyapatite are well documented. It is rapidly integrated into the human body, while at the same time the body is none the wiser as to the invasion by a foreign body, albeit a friendly invasion. Perhaps it’s most interesting property is that hydroxyapatite will bond to bone forming indistinguishable unions. 16 2.2.3. Disadvantages of Hydroxyapatite However, poor mechanical properties (in particular fatigue properties) mean that hydroxyapatite cannot be used in bulk form for load bearing applications such as orthopaedics. 2.2.4. Hydroxyapatite Coatings Coatings of hydroxyapatite have good potential as they can exploit the biocompatible and bone bonding properties of the ceramic, while utilizing the mechanical properties of substrates such as Ti6Al4V and other biocompatible alloys. While the metallic materials have the required mechanical properties, they benefit from the hydroxyapatite which provides an osteophilic surface for bone to bond to, anchoring the implant and transferring load to the skeleton, helping to combat bone atrophy. 2.2.4.1. Coating Techniques: There are several methods for coating of HAp in the table 2.1 some of them was shown: Table 2.1: Coating techniques of Hydroxyapatite Technique Thickness Advantages Disadvantages Dip Coating 0.05- Inexpensive Requires 0.5mm Coatings Thermal coat sintering applied temperatures quickly Can high expansion complex mismatch substrates Sputter Coating 0.02-1µm Uniform thickness substrates coating Line of sight technique on flat Expensive Time consuming Cannot coat complex substrates Produces coating 17 Technique Hot Thickness Advantages Pressing 0.2- and Disadvantages Produces dense coatings Hot 2.0mm HP cannot coat complex substrates Isostatic High temperature required Pressing Thermal expansion mismatch Elastic property differences Expensive Removal/Interaction of encapsulation material Electrophoretic 0.1- Uniform Deposition thickness free coatings Rapid deposition rates Requires 2.0mm Can coat coating Difficult to produce crack- high sintering complex temperatures substrates Thermal 30-200µm High deposition rates Spraying Line of sight technique High temperatures induce decomposition Rapid cooling produces amorphous coatings Sol-Gel <1µm Can coat complex Some processes shapes Low processing controlled require atmosphere temperatures Relatively processing cheap as coatings are Expensive raw materials very thin 18 2.3. Related Works on usage of hydroxyapatite: 2.3.1. Bioactive coatings on 316L stainless steel implants: Bioactive hydroxyapatite has a substantial interest because of its chemical similarity to the calcium phosphate minerals in biological hard tissue, and its ability to form a strong chemical bond with bone1. But the fracture toughness of the hydroxyapatite ceramics does not exceed the value of about 1 MPa.m1/2. Therefore, the hydroxyapatite ceramic materials cannot be used as heavy-loaded implants, such as artificial bone or teeth. Metallic implants (316L stainless steel, titanium, Ti-6Al-4V, etc.) are having high strength and fracture toughness, but their bonding ability to bone tissue is very low. In order to obtain bioactive and strong materials, the formation of hydroxyaptite on an implant with good mechanical properties is considered a good approach. Biphasic calcium phosphate coating is preferred when implant resorbability is desired. Coatings of hydroxyapatite on metallic implants have been prepared by a variety of techniques, including plasma spraying, sol-gel, r.f sputtering, detonation gun coating, high velocity oxy-fuel coating, electrophoretic deposition, laser ablation, hydrothermal and biomimetic methods. At present, plasma spraying is the most commonly used method for preparation of the hydroxyapatite coatings. However, plasma-spraying method suffers with hydroxyapatite phase stability, lack of crystallanity and poor adhesion to the substrate. The electrophoretic methods have problems with poor adhesion and formation of other phases. The r.f sputtering suffers with amorphous nature of the coating material. This paper describes a simple dipcoating method, which produces a thin and adherent HA and BCP coatings on 316L stainless steel. Therefore for conclusion: 19 a) Dip coating is a simple method to produce hydroxyapatite or biphasic calcium phosphate coating on stainless steel substrates. b) The dense, fracture free coating can improve adhesion with the substrate and also acts as barrier layer between implant surface and body fluids. c) By dip-coating method, it’s possible to obtain a very thin coating of thickness 5-10 (m for both hydroxyapatite and biphasic calcium phosphate coatings on 316L stainless steel. 2.3.2. Hydroxyapatite coating on a cobalt base alloy by investment casting: A cobalt alloy was cast into preheated molds previously coated with hydroxyapatite powder. Two molds were used, one made of investment material and the other of pure graphite. Selected samples were heat treated. Both heat and non heat treated samples were immersed in simulated body fluid for 21 days at 37 _C. A ceramic layer, identified as hydroxyapatite, was formed on all the samples. A thicker layer was formed on the sample cast into the investment mold without heat treatment. A chemical interaction between the investment mold and hydroxyapatite takes place leading to a higher in vitro bioactivity.A cobalt base alloy was cast into a mold in which the cavities were coated with hydroxyapatite powder. Bioactive ceramic particles were embedded on the surface of the alloy. A dense and homogeneous bonelike apatite layer was formed on the as cast cobalt alloy surface after 21 days of immersion in SBF. A decrease in bioactivity was observed in the heat treated samples due to the decomposition of HA. 20 Table4.1: Summarized related works on HAp coatings Authors Name Title & Methods Conclusions 1- J.C. Escobedo , J.C. Hydroxyapatite coating on A decrease in Ortiz, J.M. Almanza, D.A. a cobalt base alloy by bioactivity was observed in Corte´s, Scripta Materialia investment casting the heat treated samples vol.54 (2006) due to the decomposition of HAp. 2- TuantuanliI, Junhee Lee Hydroxyapatite coating by The dipping method is an ,Takayuki Kobayashi, dipping method, and bone effective Hideki Aoki Journal of bonding strength technique for preparing HA coated material science, Materilas titanium materials in Medicine, 7 (1996) 355 complicated shapes. The 357 material had with good biocompatibility and may be effective for use as prostheses. 3- A. De Carlos et. al. J In vitro testing of The calcium phosphate Mater Science: Mater Med Nd:YAG laser processed coatings obtained by the 17:1153–1160 (2006) calcium coatings phosphate Nd:YAG laser cladding technique showed a behaviour similar to the reference materials, Ti- 6Al-4V alloy and CaP coatings produced by plasma spray, respective to cell morphology(SEM observations),cell proliferation (Alamar Blue assay) and cytotoxicity of extracts (MTT assay). 21 Authors Name Title & Methods Conclusions 4- Bunyamin Aksakal C. Bioceramic dip-coating on Cheap, Hanyaloglu,Sci: easy, repeatable Mater Ti–6Al–4V and 316L SS with high production rate Med 2007 implant materials of bioceramic coatings of the Ti6Al4V and 316L SS implant materials achieved by are using a dipping method 5- D.A. Cortes, A.A. Biomimetic apatite Results indicate that the Nogiwa , J.M. Almanza , coating onMg-PSZ/Al2O3 biomimetically treated S. zirconia composites, by Ortega. Materials composites. Effect of the Letters 59 1352– 1355 immersion method using the re-immersion (2005) method, show a high in vitro bioactivity and may exhibit a bonebonding ability through the apatite layer. 6- H.H. Rodrı´guez H.H. Electrophoretic deposition The apatite formed on the Rodrı´guez, G. Vargas, of bioactive wollastonite porcelain coating after the D.A. Corte´s. Ceramics and porcelain– dissolution International (2007) wollastonite coatings on wollastonite layer, seemed 316L stainless steel to be strongly attached. of the The heat treatment of the samples after electrophoretic deposition has a positive effect on the bioactivity, since no apatite was formed on the non-sintered wollastonite coated samples. 22 Authors Name 8- Jie Weng Biomoterials (1997) et. Title & Methods al. Formation and vol.18 characteristics of the Conclusions The HA structure of the heated coating apatite layer on plasma- critical for sprayed hydroxyapatite nucleate in SBF without coatings in simulated sufficient dissolution of the body fluid coating to increase the local apatite supersaturation calcium ions. is and not to of phosphate CHAPTER 3 METHODOLOGY AND EXPERIMENTAL PROCEDURE: 3.1. Introduction: In this project the quality of adhesion of Hydroxyapatite on the investment casting mould was investigated. Viscosity of HAp-water mixture, dewaxing temperature and firing temperature had the main effect on the quality of adhesion of HAp on to the inner layer of investment casting mould. so investigation through different viscosities , dewaxing and firing temperature and finding the differentiation of them was the main objective, to find out the best HAp-water mixture , dewaxing temperature and firing temperature for this purpose. The project will be conducted within the below boundaries: i. 3different hydroxyapatite slurry viscosities (3 different viscosities) ii. 3different dewaxing temperature (3 different temperatures) iii. Shell investment casting mould with 5 layers thickness will be employed. For this purpose at first it needed to design appropriate parts and belongings of them to do sound casting and obtain correct results. 24 3.2 Methodology and Experiment i. Preparing the raw materials and equipments which are needed for investment casting and testing the prepared specimen, for this reason enough raw materials was prepared, the materials were: a. Hydroxyapatite as a main material that was used for coating on to the investment casting mould. b. Hyfill wax was used as the material of the pattern for investment casting. c. Aluminum rod that was used for making appropriate mould for making wax patterns. d. Zircon powder as the base material of investment casting mould e. Stucco in 2 different sizes fine () and coarse. f. Mineral water as a solvent for HAp. g. Cooking oil And necessary equipments for the experiment were: a. Mixer machine b. appropriate containers c. Ordinary lathe machines for manufacturing the Aluminum mould for making patterns. d. Drill machine e. Firing furnace (that is used for both firing and dewaxing process) it should heat up to 1000 C. ii. After preparing needed equipments and materials designing the experiment is the significant point for doing the project. The experimental process was started from designing an appropriate mould for manufacturing of the wax patterns. Then its go through coating the patterns with HAp-water mixture, ceramic mould making and dewaxing, finally chosen moulds were fired in the furnace for 1 hour in 600 C to increase the strength of the mould and supervise the effect of the heat on the coated 25 HAp layer. For obtaining the results 3 kinds of test were applied. XRD EDAX 3Dmicroscope. The whole process of the project was shown in a flowchart in figure 3.1. START Checking Requirements Preparing mould for making patterns Making Wax Patterns Coating patterns with HAp Making Ceramic Mould Dewaxing the moulds 100 C 200 C 300 C Choosing the best Dewaxed 300 C Fire the moulds up to 600 C Doing the tests XRD EDAX Supervise Figure 3.1: Flowchart of the process 3Dmicroscop END 26 Defined objectives of the project were to investigate what is the best dewaxing temperature for dewaxing the moulds and also find out which of the HApwater mixture viscosities would be the best one for coating the inner layer of the moulds. 3.2.1 Design: 3.2.1.1 Pattern Design: At the first stage of experiment the shape of the pattern for the mould designed. Regarding to the limitations of the project which were i. High price of HAP ii. Previous experiment iii. Desired characteristics iv. Equipment availability v. Simplifying the experiment vi. Number of required samples for different testes, etc The patterns consist of a pouring cup and three cylindrical samples were designed. Dimensions and general view of the pattern is shown in figure3.2 for making this pattern from the wax Aluminum mould was designed. This mould has ability to make three wax samples and a pouring cup. According to high price of HAp and objective of the project the size of the samples were minimized Regarding to the even condition of the coating the samples were designed in cylindrical shape. For increasing the number of samples, easier casting and to overcome the defects that may occur during the solidification process the pouring cup with stated dimensions designed. This part provides the pouring cup for uniform molten metal flow in to the samples as well. 27 In accordance with previous investment casting experiments and estimated thickness of the ceramic mould which is based on the number of layers, the arrangement of the samples and the distance between them were calculated. and was shown in figure 3.2. Indeed, based on the shape of the patterns for investment casting, it was required to prepare a mould for pattern fabrication. 17mm 20mm 15mm Figure 3.2: Drawing of a sample 3.2.1.2 Design of Wax mould: The design of considered mould was consisted of separation lines of different part of the mould, tapering, and easy final releasing of the patterns from the mould. The mould was designed in three parts for the purpose of even heat removal and good surface finish. The mould for wax pattern production consisyed of three parts; the Base which was met to produce a uniform surface finish of the wax pattern at the bottom of the samples. Middle part that contains three desired cylindrical samples. 28 The upper part separated into two parts for easy remove of the patterns from the mould. The drawings of each part are shown in figures 3.3-3.6. Figure 3.3 Base part of pattern mould 29 Figure 3.4 Middle part of the pattern mould Figure 3.5 Top part of the pattern mould 30 3.2.2 Mould Fabrication Regarding to prepared drawings and available materials, Aluminum was selected. Available materials for making the mould were aluminum and Stainless steel. Regarding to the melting temperature of the wax as the base material of the patterns, high thermal resistance of the mould material was not considerable. Furthermore easy machining characteristics for rapid and accurate machining with better final surface finish comparing to stainless steel were the key points for selecting aluminum rather than stainless steel. The raw material was an aluminum rod which was cut in 63mm diameter and 100mm height. This work piece was cut into three parts that each of them showed in the drawings. For machining of this work piece a conventional lathe machine was used. The machine tool was normal HSS regarding to the machining condition and base material which was Aluminum. During machining the surface finish and accuracy of the work piece was supervised regarding to precision casting of the wax and easy releasing of samples from the mould. 31 Middle part Top part Base part Figure 3.6 Disassembled pattern mould Figure3.7. Assembled mould for wax patter 3.2.3 Wax pattern manufacture On the subject of the characteristics of the wax that was applied to make investment casting mould, the melting temperature of the wax is around 100 32 centigrade degree. Therefore simple electric heater was a good option for melting the wax. A simple container made from aluminum was used as a pot for the melting of the wax. Solid clean pieces of wax were melted in the container by means of the electric heater. The electric heater adjusted on the 250 centigrade degree. For easy removal and releasing of the wax pattern form the mould, a good lubricant was needed. For this purpose cooking oil would be a good selection, because of its characteristics as a lubricant which is not toxic, and inflammable at the wax melts temperature. After coating inner parts of the mould with the cooking oil (Corn Oil), the melted wax was poured into the mould to make the required pattern with the desired shape. Also a hanging grip is put onto the top surface of the molten wax, so that when it solidifies it is easy to hang the patterns. Hanging the samples is required for next processes especially during coating process and ceramic mould making process steps. Solidification of the wax inside the mould takes around 15 minutes time. For making the solidification faster water was used as an extra cooler. Regarding to the mixing of water and molten wax while using water as a coolant, it should be used carefully. Therefore, the surface layer of the wax pattern which is in contact with the air should be solidified first otherwise the water can penetrate into the wax and form a nonhomogeneous mixture. Then the mould can be put into the cold water container for final and complete cooling process. A solidified wax pattern was shown in figure After removing wax pattern from the mould, pattern was hanged from its hanging part for further use and next levels. 33 Figure 3.8 Solid wax patterns inside the mould Wax Pattern Figure 3.9 Wax patterns hanged for drying 3.2.4. Ceramic mould fabrication: This stage consists of several stages those are respectively: 34 i. Preparing the wax samples for coating of hydroxylapatite. ii. Preparing 3 different Hydroxyl apatite viscosities for coating. iii. Coating the wax samples with hydroxylapatite. iv. Preparing the slurry for making the shells of investment casting mould. v. Fabrication of Ceramic mould, stuccoing, and final sealing 3.2.4.1. Preparing Wax Samples: In this step, wax samples were washed by a simple detergent to remove extent oil remained from the surface of the samples in wax casting stage. Remaining of oil on the surface of the samples will affect the stickiness of the first coating layer and the quantity of hydroxylapatite that would be coated on the surface. For washing the samples enough amount of detergent was mixed with water in an appropriate container, and then samples were washed inside the container. Subsequently, enough time should be taken until all the samples become completely dried. 3.2.4.2. Preparing Hydroxylapatite for coating After preparing all the samples, sufficient amount of Hydroxylapatite mixed with water. The mixing process is described as following: As there was no information about the mixture ratio of Hydroxylapatite and water to obtain the desired viscosity, a step by step method was chosen. In this method the volume of water that was enough for soaking a sample or pattern into the mixture of water and HA applied then step by step HA added to the water and the mixture was stirred up continuously. Besides, the viscosity of the mixture was measured concurrently until the desired viscosity was achieved. The desired 35 viscosities for this purpose were 5, 7.5 and 10 seconds as previously mentioned in the scopes of the project. For measuring the viscosities a Zhan’s cup was used. Obtaining the viscosities more than 10 seconds was not applicable because of creamy characteristic of HA-water mixture. Regarding to this characteristic of HAwater mixture, using the mixing machines was not considered suitable as well. Indeed, the mixture was stirred manually and by hand during the preparation of the mixture to obtaining the desired viscosities. For coating of HAp-water mixture on the samples, there were several methods. However, in this project soaking in to the HAp-water mixture was applied. Therefore samples were soaked into different viscosities for 30 seconds to make sure enough HAp was adhered to the samples, and then all of them hanged for about 2 hours to be dried completely. In figure 3.10 mixing process of HAp and water was shown in figure 3.10. Figure 3.10 Mixing HAp and mineral water 36 3.2.4.3-Coating of samples There are different ways to coat HAp on to the samples i.e. as it was illustrated in literature review… applied paint brush to coat samples with HAp-water mixture. As investment casting was applied in this project, the samples were dipped into the mixture of water and HAp. In this case, soaking of samples into the Hp-water mixtures was done. All the samples were divided to 3 groups each group contains 9 samples as it was shown in the table below. Initially the samples were dipped into the mixture for 30 seconds and then they were removed and hanged for about 2 hours to be almost dried. In case of any defects or crakes the samples were dipped again to make sure that the coating is sound. Based on the preliminary experiments it was concluded that complete dryness of Hap coating layer will result in some defects which is mostly crakes. These crakes can affect the thickness and quality of Hap coating layer which is not good for the purpose of this process and experiment. Therefore incomplete dryness is appropriate method to overcome this problem and prepare the samples for slurry coating. In figure 3.11 measuring the viscosity of HAp-water mixture was shown. 37 Figure 3.11 Measuring the viscosity of HAp-Water mixture 3.2.4.4. Slurry preparation To prepare the investment casting slurry in this project Zircon powder and Colloidal Silica were mixed together with specific viscosities (20,25 and at last coating 20 again). At first Colloidal Silica was poured in to the container and Zircon powder was mixed gradually while the mixture was continuously stirred by a mixer to make homogenous slurry and even properties in the whole slurry. Figure 3.12 was shown the mixing process. The appropriate viscosity was achieved after several tests. The measurement tool was a standard Zhan’s cup with a 5 millimeter hole. To measure the viscosity the cup was filed up of the slurry and the time for pour out of slurry was measured by a chronometer. 38 Figure 3.12 Preparing ceramic slurry from colloidal silica and zircon powder. 39 3.2.4.5. Investment casting mould making The mould was built with 7 layers. For the first layer and sealing layer (last layer) higher viscosity was selected. Desired viscosity was 25 seconds. Higher viscose slurry will take less time to become dry and have higher stickiness due to less flow ability. Therefore it provides better overall quality and sufficient thickness for the first layer and last layer. For the back up coats layers the viscosity of 20 seconds was selected. It will provide enough stickiness for stucco particles and sufficient thickness as a layer of the mould as well. They were 5 back up coats slurry layers which all were completely covered with the stucco. They were two available stucco sizes; fine and coarse. Based on the initial experiments the combination of fine and coarse was selected. After the first layer the fine stucco size was applied to cover the first middle slurry layer and followed by coarse stucco size for the second layer. This sequence was employed for all 5 middle layers which mean that first, third and fifth middle layers were covered with fine stucco while second and fourth middle layers were covered with coarse stucco. This mixture provided two important properties which are good strength and good permeability that are resulted from fine and coarse stucco respectively. Indeed there were seven layers with the following order: HAp coat, slurry, five layers back up coats, and final sealing coat. The making of the investment casting mould used for the investigation was shown in figures 3.13 to 3.16. 40 Figure 3.13: Coating wax samples with ceramic slurry Figure 3.14: Coated wax pattern with slurry 41 Figure 3.15: Moulds after stuccoing Figure 3.16: Moulds after final coating 42 3.2.5. Effect of dewaxing temperature: 3.2.5.1. Introduction Three dewaxing temperatures were chosen 100 C, 200 C and 300 C to determine it’s effect on HAp coatings. Dewaxing was conducted in an electric resistance furnace prior to dewaxing seven holes were drilled on the wax at the pouring cup Figure3.17. Prior to insert the moulds into the furnace; the furnace was heated to the required temperature, once the temperature needed, the moulds were placed in the arrangement shown in figure 3.18. the moulds were removed from the furnace after 1 hour. The procedure was repeated for the temperatures of 200 and 300 C Considering the scope of the project three dewaxing temperatures were tested. Generally the dewaxing temperature for investment casting is between 100 to 300 degrees centigrade. Selected temperatures were 100,200 and 300 degrees centigrade to find out the appropriate dewaxing temperature for the process. 3.2.5.2. Dewaxing Dewaxing process was done in resistance furnace with the following specifications. Figures: The moulds were put upside down in the furnace for one hour to melt the wax and for wax removal from the moulds. Regarding to initial test moulds the expansion of the wax during dewaxing process leaded to mould cracks, to prevent this defect there are several solutions, in this project a new method was applied to overcome the problem. New method was reducing the amount of the wax inside the mould. For this purpose the wax was removed from the open head of the mould by drilling. As it was shown in figures5.12, 6 or 7 holes were drilled in the pouring cup of the mould with the appropriate depth. Depths of the holes were around 1.5 cm to assure that they 43 will not reach the mould’s body. The moulds were not heated up from environment temperature to the dewaxing temperature. Therefore the furnace turned on and heated up to desired temperature and then the moulds were put in the furnace for one hour to remove the wax. Figure 3.17 Making holes inside the wax Figure 3.18 Moulds inside the furnace 44 3.2.6 Firing process: Dewaxed moulds must be fired for several purposes. The most important objectives of firing process specifically for this project include complete removal of remain wax in the moulds and increasing the strength of the moulds by sintering the layers. Firing process was carried out with the same furnace that had been used for dewaxing process. In addition, the process was also similar to the dewaxing process which means that the furnace was heated up to the selected firing temperature and the moulds were put in the furnace for one hour to perform the firing process. Regarding to defined scope and objectives of this project three different firing temperatures were selected. In addition this selection was regarding to the properties and characteristics of applied bio active material (Hydroxyl apatite). The structural phase of this material changes according to specific temperature range.The starting temperature for phase modification is 800 degree centigrade. Hydroxyl apatite is thermally unstable compound, decomposing at temperature from about 800 to 1200 degree centigrade depending on its stoichiometry. Based on this fact and the conditions of this research three different firing temperatures were selected including 600, 800 and 1000 degree centigrade. These temperatures were selected to study the effect of different temperatures below the range, above the range and exactly at the border of the range. Therefore the behavior of the Hydroxyl apatite with different firing temperature can be studied.but for the purpose of understanding which viscosity and dewaxing temperature is the best selection for moulds only 600 degree centigrade was applied. 3.2.7. Tests The final part of the project includes test results and discussions about them to evaluate the achieved results of the experiments. These results will clarify the best 45 combination of experimental factors for better and effective casting results that depends on the factors that are needed for production of medical implants in future. The experimental tests consist of XRD (X Ray Diffraction), EDAX (Energydispersive X-ray spectroscopy), SEM (Scanning Electron Microscope), 3D microscope imaging. XRD: X-ray scattering techniques are a family of non-destructive analytical techniques which reveal information about the crystallographic structure, chemical composition, and physical properties of materials and thin films. These techniques are based on observing the scattered intensity of an x-ray beam hitting a sample as a function of incident and scattered angle, polarization, and wavelength or energy. EDAX: Energy dispersive X-ray spectroscopy (EDS, EDX or EDXRF) is an analytical technique used for the elemental analysis or chemical characterization of a sample. As a type of spectroscopy, it relies on the investigation of a sample through interactions between electromagnetic radiation and matter, analyzing x-rays emitted by the matter in response to being hit with the electromagnetic radiation. Its characterization capabilities are due in large part to the fundamental principle that each element has a unique atomic structure allowing x-rays that are characteristic of an element's atomic structure to be indentified uniquely from each other. To stimulate the emission of characteristic x-rays from a specimen, a high energy beam of charged particles such as electrons or protons, or a beam of x-rays, is focused into the sample being studied. At rest, an atom within the sample contains ground state (or unexcited) electrons in discrete energy levels or electron shells bound to the nucleus. The incident beam may excite an electron in an inner shell, ejecting it from the shell while creating an electron hole where the electron was. An electron from an outer, higher-energy shell then fills the hole, and the difference in energy between the higher-energy shell and the lower energy shell is released in the form of an x-ray. The x-ray released by the electron is then detected and analyzed by 46 the energy dispersive spectrometer. These x-rays are characteristic of the difference in energy between the two shells, and of the atomic structure of the element form which they were emitted. For performing XRD, EDAX and 3Dmicroscope tests, moulds were broken carefully (Figure 3.19) to prepare required samples for each test. For XRD test the required amount of powder were scratched from the adhered HAp of the mould wall (Figure 3.20). In addition for EDAX and SEM tests a small part of the mould with HAp on the wall was selected. This selection was because of the required size for EDAX and SEM machine. Sizing for EDAX and SEM samples were done by technicians of the machines at UTHM, Batu Pahat. For 3D microscope the down part of the mould were taken which had vertical walls of the mould with HAp on it this was selected for better showing of adhesion of the HAp to the moulds wall.(Figure 3.19) 47 Figure 3.19 Broken parts of the samples for tests. Figure 3.20 Prepared powder for XRD test CHAPTER4 RESULT AND DISCCUSION As mentioned above four kinds of tests had been performed to analyze the samples and verify the results with available standards. The results were categorized based on the tests that were carried out. At first XRD results are presented and discussed to confirm the existence of suitable chemical compositions in the produced samples. Next EDAX results had been issued to verify the existence of necessary elements and confirm the desired ratio of required elements. Moreover SEM results were presented to show the micro structure of the adhered HAp on the inner surface of the mould. Finally 3D microscope images had been taken to show the thickness and stickiness of HAp to the mould. 4.1. XRD Result To specify a reference and understanding the initial available components in the considered base materials XRD test was performed on all three kinds of the materials. Provided materials for this project were from three different suppliers that are Fluka, Granumas, Plasma Tech Co. Figures 4.1 to 4.3 show the XRD results of Fluka, Granumas, Plasma Tech Co. respectively. 18000 17000 16000 15000 14000 13000 12000 11000 Lin (Counts) Figure 4.1 XRD result for Fluka Fluka 10000 9000 8000 7000 6000 5000 4000 3000 2000 1000 0 4 10 20 30 40 50 60 2-Theta - Scale Fluka - File: Fluka.raw - Type: 2Th/Th locked - Start: 3.000 ° - End: 129.999 ° - Step: 0.020 ° - Step time: 15.5 s - Temp.: 25 °C (Room) - Time Started: 10 s - 2-Theta: 3.000 ° - Theta: 1.500 ° - Chi: 0.00 ° - Ph Operations: Background 1.000,1.000 | Import 00-024-0033 (D) - Hydroxylapatite - Ca5(PO4)3(OH) - Y: 8.37 % - d x by: 1. - WL: 1.5406 - Hexagonal - a 9.43200 - b 9.43200 - c 6.88100 - alpha 90.000 - beta 90.000 - gamma 120.000 - Primitive - P63/m (1 00-055-0592 (*) - Hydroxylapatite, syn - (Ca)10(PO4)6(OH)2 - Y: 22.36 % - d x by: 1. - WL: 1.5406 - Hexagonal - a 9.41890 - b 9.41890 - c 6.88270 - alpha 90.000 - beta 90.000 - gamma 120.000 - Primitive 00-009-0432 (I) - Hydroxylapatite, syn - Ca5(PO4)3(OH) - Y: 7.20 % - d x by: 1. - WL: 1.5406 - Hexagonal - a 9.41800 - b 9.41800 - c 6.88400 - alpha 90.000 - beta 90.000 - gamma 120.000 - Primitive - P63/ 00-003-0747 (D) - Hydroxylapatite - Ca10(PO4)6(OH)2 - Y: 5.50 % - d x by: 1. - WL: 1.5406 00-001-1008 (D) - Hydroxyapatite - Ca10(OH)2(PO4)6 - Y: 7.89 % - d x by: 1. - WL: 1.5406 - 49 18000 17000 16000 15000 14000 13000 12000 11000 Lin (Counts) Figure 4.2 XRD result granumas Granumas 10000 9000 8000 7000 6000 5000 4000 3000 2000 1000 0 3 10 20 30 40 50 60 2-Theta - Scale Granumas - File: Granumas.raw - Type: 2Th/Th locked - Start: 3.000 ° - End: 69.990 ° - Step: 0.020 ° - Step time: 15.5 s - Temp.: 25 °C (Room) - Time Started: 10 s - 2-Theta: 3.000 ° - Theta: 1.500 ° - Chi: 0.00 ° - Phi Operations: Background 1.000,1.000 | Import 00-024-0033 (D) - Hydroxylapatite - Ca5(PO4)3(OH) - Y: 15.63 % - d x by: 1. - WL: 1.5406 - Hexagonal - a 9.43200 - b 9.43200 - c 6.88100 - alpha 90.000 - beta 90.000 - gamma 120.000 - Primitive - P63/m (176) - 2 00-009-0432 (I) - Hydroxylapatite, syn - Ca5(PO4)3(OH) - Y: 14.33 % - d x by: 1. - WL: 1.5406 - Hexagonal - a 9.41800 - b 9.41800 - c 6.88400 - alpha 90.000 - beta 90.000 - gamma 120.000 - Primitive - P63/m (176) 00-055-0592 (*) - Hydroxylapatite, syn - (Ca)10(PO4)6(OH)2 - Y: 42.83 % - d x by: 1. - WL: 1.5406 - Hexagonal - a 9.41890 - b 9.41890 - c 6.88270 - alpha 90.000 - beta 90.000 - gamma 120.000 - Primitive - P63/m ( 00-003-0747 (D) - Hydroxylapatite - Ca10(PO4)6(OH)2 - Y: 8.52 % - d x by: 1. - WL: 1.5406 00-001-1008 (D) - Hydroxyapatite - Ca10(OH)2(PO4)6 - Y: 10.19 % - d x by: 1. - WL: 1.5406 - 50 18000 17000 16000 15000 14000 13000 12000 11000 Lin (Counts) Figure 4.3 XRD result Iran Iran 10000 9000 8000 7000 6000 5000 4000 3000 2000 1000 0 4 10 20 30 40 50 60 2-Theta - Scale Iran - File: iran.raw - Type: 2Th/Th locked - Start: 3.000 ° - End: 69.990 ° - Step: 0.020 ° - Step time: 15.5 s - Temp.: 25 °C (Room) - Time Started: 10 s - 2-Theta: 3.000 ° - Theta: 1.500 ° - Chi: 0.00 ° - Phi: 0.00 ° - X: 0 Operations: Background 1.000,1.000 | Import 00-024-0033 (D) - Hydroxylapatite - Ca5(PO4)3(OH) - Y: 0.73 % - d x by: 1. - WL: 1.5406 - Hexagonal - a 9.43200 - b 9.43200 - c 6.88100 - alpha 90.000 - beta 90.000 - gamma 120.000 - Primitive - P63/m (176) - 2 00-009-0432 (I) - Hydroxylapatite, syn - Ca5(PO4)3(OH) - Y: 0.62 % - d x by: 1. - WL: 1.5406 - Hexagonal - a 9.41800 - b 9.41800 - c 6.88400 - alpha 90.000 - beta 90.000 - gamma 120.000 - Primitive - P63/m (176) 00-055-0592 (*) - Hydroxylapatite, syn - (Ca)10(PO4)6(OH)2 - Y: 1.51 % - d x by: 1. - WL: 1.5406 - Hexagonal - a 9.41890 - b 9.41890 - c 6.88270 - alpha 90.000 - beta 90.000 - gamma 120.000 - Primitive - P63/m (1 51 52 A brief summary of XRD results for base materials and available chemical compositions in these materials are listed in Table4.1 Table4.1: XRD Test Results – Reference Materials XRD Test Results – Reference Materials Material Hydroxlyapatite [Ca5(PO4)3(OH)] Hydroxlyapatite,syn [Ca10(PO4)6(OH)2] Hydroxlyapatite,syn [Ca5(PO4)3(OH)] Hydroxlyapatite [Ca10(PO4)6(OH)2] Hydroxyapatite [Ca10(OH)2(PO4)6] Material Code Fluka Granumas Plasma Tech Co. 0033 1550 2900 250 0592 4050 7600 350 0432 1400 2550 200 0747 650 1600 - 1008 1450 1850 - Regarding to exsiccating limitations that include availability, price, and appropriate chemical composition the HAp provided by Fluka was selected. It can be seen from the table that HAp from Fluka and Granumas offered required chemical compositions better than Plasma Tech and indeed Plasma Tech was rejected (Figure 204). On the other hand, with considering the price of available materials it was concluded to apply Fluka instead of Granumas. 53 XRD Results - Reference Materials 8000 7000 6000 Lin (Counts) 5000 Hydroxlyapatite - [Ca5(PO4)3(OH)] Hydroxlyapatite, syn - [Ca10(PO4)6(OH)2] Hydroxlyapatite, syn - [Ca5(PO4)3(OH)] Hydroxlyapatite - [Ca10(PO4)6(OH)2] Hydroxyapatite - [Ca10(OH)2(PO4)6] 4000 3000 2000 1000 0 Fluka Granumas Plasma Tech Co. Company Figure 4.4 XRD result for reference materials XRD test was also performed for three moulds that were fired in 600 C temperature to figure out the remaining chemical compositions of the materials. It will illustrate effect of firing temperature and viscosities result in higher amount of necessary component for medical use and next steps. In this project availability and quality of adhered HAp is the main objective. Therefore this illustration can clarify the best procedure for producing moulds. Actual XRD test results for all nine samples were shown in figures 4.5-4.7 18000 17000 16000 15000 14000 13000 12000 11000 Lin (Counts) Figure 4.5 Result of XRD for 5 s viscosity and 600 C 5S600C 10000 9000 8000 7000 6000 5000 4000 3000 2000 1000 0 4 10 20 30 40 50 60 2-Theta - Scale 5S600C - File: 5S600C.raw - Type: 2Th/Th locked - Start: 3.000 ° - End: 69.990 ° - Step: 0.020 ° - Step time: 15.5 s - Temp.: 25 °C (Room) - Time Started: 11 s - 2-Theta: 3.000 ° - Theta: 1.500 ° - Chi: 0.00 ° - Phi: 0.0 Operations: Background 1.000,1.000 | Import 00-024-0033 (D) - Hydroxylapatite - Ca5(PO4)3(OH) - Y: 8.31 % - d x by: 1. - WL: 1.5406 - Hexagonal - a 9.43200 - b 9.43200 - c 6.88100 - alpha 90.000 - beta 90.000 - gamma 120.000 - Primitive - P63/m (176) - 2 00-055-0592 (*) - Hydroxylapatite, syn - (Ca)10(PO4)6(OH)2 - Y: 19.30 % - d x by: 1. - WL: 1.5406 - Hexagonal - a 9.41890 - b 9.41890 - c 6.88270 - alpha 90.000 - beta 90.000 - gamma 120.000 - Primitive - P63/m ( 00-009-0432 (I) - Hydroxylapatite, syn - Ca5(PO4)3(OH) - Y: 7.20 % - d x by: 1. - WL: 1.5406 - Hexagonal - a 9.41800 - b 9.41800 - c 6.88400 - alpha 90.000 - beta 90.000 - gamma 120.000 - Primitive - P63/m (176) 00-003-0747 (D) - Hydroxylapatite - Ca10(PO4)6(OH)2 - Y: 6.05 % - d x by: 1. - WL: 1.5406 00-001-1008 (D) - Hydroxyapatite - Ca10(OH)2(PO4)6 - Y: 7.26 % - d x by: 1. - WL: 1.5406 - 54 18000 17000 16000 15000 14000 13000 12000 11000 Lin (Counts) Figure 4.6 result of XRD for 7.5 s viscosity and 600 C 7.5S600C 10000 9000 8000 7000 6000 5000 4000 3000 2000 1000 0 3 10 20 30 40 50 60 2-Theta - Scale 7.5S600C - File: 7.5S600C.raw - Type: 2Th/Th locked - Start: 3.000 ° - End: 69.990 ° - Step: 0.020 ° - Step time: 15.5 s - Temp.: 25 °C (Room) - Time Started: 10 s - 2-Theta: 3.000 ° - Theta: 1.500 ° - Chi: 0.00 ° - Phi: Operations: Background 1.000,1.000 | Import 00-024-0033 (D) - Hydroxylapatite - Ca5(PO4)3(OH) - Y: 5.20 % - d x by: 1. - WL: 1.5406 - Hexagonal - a 9.43200 - b 9.43200 - c 6.88100 - alpha 90.000 - beta 90.000 - gamma 120.000 - Primitive - P63/m (176) - 2 00-055-0592 (*) - Hydroxylapatite, syn - (Ca)10(PO4)6(OH)2 - Y: 13.37 % - d x by: 1. - WL: 1.5406 - Hexagonal - a 9.41890 - b 9.41890 - c 6.88270 - alpha 90.000 - beta 90.000 - gamma 120.000 - Primitive - P63/m ( 00-009-0432 (I) - Hydroxylapatite, syn - Ca5(PO4)3(OH) - Y: 5.52 % - d x by: 1. - WL: 1.5406 - Hexagonal - a 9.41800 - b 9.41800 - c 6.88400 - alpha 90.000 - beta 90.000 - gamma 120.000 - Primitive - P63/m (176) 00-003-0747 (D) - Hydroxylapatite - Ca10(PO4)6(OH)2 - Y: 4.88 % - d x by: 1. - WL: 1.5406 00-001-1008 (D) - Hydroxyapatite - Ca10(OH)2(PO4)6 - Y: 8.23 % - d x by: 1. - WL: 1.5406 - 55 18000 17000 16000 15000 14000 13000 12000 11000 Lin (Counts) Figure 4.7 Result of XRD for 10 s viscosity and 600 C 10S600C 10000 9000 8000 7000 6000 5000 4000 3000 2000 1000 0 4 10 20 30 40 50 60 2-Theta - Scale 10S600C - File: 10S600C.raw - Type: 2Th/Th locked - Start: 3.000 ° - End: 69.990 ° - Step: 0.020 ° - Step time: 15.5 s - Temp.: 25 °C (Room) - Time Started: 10 s - 2-Theta: 3.000 ° - Theta: 1.500 ° - Chi: 0.00 ° - Phi: Operations: Background 1.000,1.000 | Import 00-055-0592 (*) - Hydroxylapatite, syn - (Ca)10(PO4)6(OH)2 - Y: 20.93 % - d x by: 1. - WL: 1.5406 - Hexagonal - a 9.41890 - b 9.41890 - c 6.88270 - alpha 90.000 - beta 90.000 - gamma 120.000 - Primitive - P63/m ( 00-024-0033 (D) - Hydroxylapatite - Ca5(PO4)3(OH) - Y: 7.10 % - d x by: 1. - WL: 1.5406 - Hexagonal - a 9.43200 - b 9.43200 - c 6.88100 - alpha 90.000 - beta 90.000 - gamma 120.000 - Primitive - P63/m (176) - 2 00-009-0432 (I) - Hydroxylapatite, syn - Ca5(PO4)3(OH) - Y: 6.44 % - d x by: 1. - WL: 1.5406 - Hexagonal - a 9.41800 - b 9.41800 - c 6.88400 - alpha 90.000 - beta 90.000 - gamma 120.000 - Primitive - P63/m (176) 00-003-0747 (D) - Hydroxylapatite - Ca10(PO4)6(OH)2 - Y: 6.10 % - d x by: 1. - WL: 1.5406 00-001-1008 (D) - Hydroxyapatite - Ca10(OH)2(PO4)6 - Y: 8.33 % - d x by: 1. - WL: 1.5406 - 56 57 In table 6.2 the results were summarized for better comparison. To separate the results for each viscosity, the results of XRD test for each viscosity 600 C firing temperature are shown in Figures 214-219. Table 4.2: XRD results for different viscosities Dewaxing 300 Temperature (°C) Viscosity (s) 5 7.5 10 600 600 600 0033 1500 1000 1400 0592 3500 2450 3750 0432 1350 1050 1250 0747 1100 600 1200 1008 1400 1500 1500 Firing Temperature (°C) Material Hydroxlyapatite [Ca5(PO4)3(OH)] Hydroxlyapatite,syn [Ca10(PO4)6(OH)2] Hydroxlyapatite,syn [Ca5(PO4)3(OH)] Hydroxlyapatite [Ca10(PO4)6(OH)2] Hydroxyapatite [Ca10(OH)2(PO4)6] Material Code Figures 4.8, 4.9 and 4.10show the intensities of desired chemical compositions of three different viscosities of HAp slurry. It can be realized that in 600 degree centigrade the highest amount of these desired components is available in 10 s viscosity. 58 Material Amount vs. Temperature - 5s Viscosity 4000 3500 3000 Lin (Counts) 2500 Hydroxlyapatite - [Ca5(PO4)3(OH)] Hydroxlyapatite, syn - [Ca10(PO4)6(OH)2] 2000 Hydroxlyapatite, syn - [Ca5(PO4)3(OH)] Hydroxlyapatite - [Ca10(PO4)6(OH)2] Hydroxyapatite - [Ca10(OH)2(PO4)6] 1500 1000 500 0 600 Firing Temperature (C) Figure 4.8: Material amount vs. temperature in 5 s viscosity Material Amount vs. Temperature - 7.5s Viscosity 3000 2500 Lin (Counts) 2000 Hydroxlyapatite - [Ca5(PO4)3(OH)] Hydroxlyapatite, syn - [Ca10(PO4)6(OH)2] 1500 Hydroxlyapatite, syn - [Ca5(PO4)3(OH)] Hydroxlyapatite - [Ca10(PO4)6(OH)2] Hydroxyapatite - [Ca10(OH)2(PO4)6] 1000 500 0 600 Firing Temperature (C) Figure 4.9: Material vs. Temperature 7.5 viscosity 59 Material Amount vs. Temperature - 10s Viscosity 4000 3500 3000 Lin (Counts) 2500 Hydroxlyapatite - [Ca5(PO4)3(OH)] Hydroxlyapatite, syn - [Ca10(PO4)6(OH)2] 2000 Hydroxlyapatite, syn - [Ca5(PO4)3(OH)] Hydroxlyapatite - [Ca10(PO4)6(OH)2] Hydroxyapatite - [Ca10(OH)2(PO4)6] 1500 1000 500 0 600 Firing Temperature (C) Figure 4.10: Material amount vs. temperature 10s viscosity 4.2 EDAX result The purpose of EDAX test is to specify the structural elements of the adhered HAp to the wall of the mould. This test will clarify the changes of the elements during the firing process regarding to the amount of the material adhered to the mould from different quantity of viscosity. The main elements that are study in this EDAX test are Phosphor and Calcium. These elements are the base and most significant components of HAp. The amount ratio of these elements is a key issue to specify the existence and phase of HAp. At first, original base materials which were provided by the three suppliers were tested. The results of these tests had beneficial conclusions regarding to the standard ratio between main elements of HAp. The most desirable ration of Calcium and Phosphate is 1.67 which shows the presence of Hydroxyapatite. In addition, the Ca/P ratio of 1.5 and 2 proves the presence of TCP (Tri-Calcium Phosphate) and TTCP respectively. Indeed these results would support key points for analyzing the 60 base materials and finally to select the best material between these three and consider it as a reference to study the changes according to firing temperatures. The results of EDAX tests for base materials are listed in Table 203. According to achieved EDAX results, it can be obviously understood that Fluka provides the best base material for the purpose of this project and also medical uses based on proven ratios reported in previous papers. This is due to the average ratio of the Ca/P which must vary from 1.5 to 2. By looking at the EDAX results of two other companies, they show higher average ratio of Ca/P that can not be accepted. Also these results along with XRD results confirm the better quality of Fluka’s Hydroxyapatite. Table 4.3 EDAX results for 600 C temperatures EDAX Test Results – Actual Samples, Fluka Dewaxing 300 Temperature (°C) Viscosity (s) Firing Temperature (°C) Test 5 7.5 10 600 600 600 Element 1 Ca/P Ratio 1.87616 2.15128 1.90722 2 Ca/P Ratio 1.67288 1.95871 1.85714 3 Ca/P Ratio 1.4548 1.91933 1.75 1.66795 2.00977 1.83812 Ca/P Ratio Average 61 Next, as it was necessary to confirm the existence of these principal elements after the firing process, all nine samples were taken for EDAX test. The results are shown in Table 6.3. By taking a glance at the results of the EDAX samples, it can be easily understood that the experimental conditions are well considered due to the existence and logical ratio of Ca/P. Most of the calculated Ca/P ratios can be found in the standard and desirable range. On the other hand, there are a few number of test results that do not show the appropriate ratio of Ca/P. However, by considering all the recognized elements in the result, it can be understood that it is the error of the technicians or some other environmental error due to the existence of high amount of Zircon and Silicon which means that the wall of the mould was taken for the test instead of HAp layer adhered to the wall. In conclusion, all other accurate test results are in the standard range of Ca/P ratio which is from 1.5 to 2. Table 4.4. 62 Table 4.4: EDAX Test Results – Actual Samples, Fluka EDAX Test Results – Actual Samples, Fluka Dewaxing(°C) Test 300 Viscosity (s) 5 7.5 10 Firing Temp(°C) 600 600 600 Be 4.33 0 32.48 C 1.81 2.92 0.23 Si 0.9 0 0.01 P 17.07 18.31 1.94 Ca 32.026 39.39 3.7 Zr 0 0 0 Be 0 5.35 35.91 C 3.14 1.05 0 Si 0 0 0 P 20.91 16.71 0.07 Ca 34.98 32.73 0.13 Zr 0 0 0 Be 0 0 35.9 C 2.01 1.9 0 Si 1.96 0 0 P 21.68 19.71 0.08 Ca 31.54 37.83 0.14 Zr 0 0 0 Element 1 2 3 1 Ca/P Ratio 1.87616 2.15128 1.90722 2 Ca/P Ratio 1.67288 1.95871 1.85714 3 Ca/P Ratio 1.4548 1.91933 1.75 Ca/PRatioAverage 1.66795 2.00977 1.83812 63 4.3 3D microscopes: One of the main objectives of the project is measuring the thickness of HAp layer adhered to the inner wall of the mould. For this purpose 2D microscope was needed but for applying this kinds of microscopes the surface of the detecting samples should be in good finishing condition. Therefore proper surface finishing is needed but because of brittle manner of adhered HAp, surface finishing is not applicable. So 3Dmicroscope will be a good choice that can detect the surface. 3d microscope pictures were taken to prove the adherence of HAp on to the walls of the mould; these pictures are shown in figure 6.11. In all of these samples a layer of HAp with minimum thickness of 0.5mm can be detected. Indeed, this confirms the validity of investment casting method for coating of HAp and its existence on the walls of the moulds to provide this bio active material on to bio metallic materials which can be produced by casting method. Figure 4.11: Adhesion of HAp to the inner layer of mould CHAPTER 5 CONCLUSION After all from the result that reached from the tests, the project objectives as were mentioned in methodology: finding the best dewaxing temperature and also find the best viscosity of HAp-water mixture for coating the inner layer of investment casting mould, obtained. For dewaxing temperature 300 C is the best because the maximum wax was removed from the investment casting mould and it dose not have any worst effect on the quality of the layers. For viscosities HAp viscosity of 5 second shows the best ratio Ca: P i.e 1.7 and fewer cracks during the process instead of 7.5 and 10 second viscosities. Regarding to this project and the other projects that were done for coating the HAp on to the different materials, in can be concluded that this method (investment casting method for coating) will be an appropriate method in the subject of coating HAp on to the metallic materials. As it discussed before this method is very cheap regarding to other coating methods and it is very simple as well and according to using simple methods control of the elements which are involved in the finale results is easier than other coating methods like plasma spraying or electro chemical methods for coating. 65 Simplifying and decreasing the cost of the process will directly affect on the price of the final product therefore it can help to make cheaper and more popular implants that will increase the life style for the people who need appropriate implants. But should be assure the accuracy of the final product and weather it is not accurate find better methods or optimize current method. A system alternate found to portray the least crack and enhanced with drilling the wax to reduce cracks. It is a new method to minimize the effect of wax expansion during the dewaxing process. This method is easy and cheap and don’t have any effect on the quality of the mould. It is possible in all the simple workshops. Regarding to hole making inside the wax pattern to assure crack-less mould during dewaxing process Indeed, the use of Investment casting method can assure the minimum requirements of the coating process of HAp (bioactive material) onto biometallic materials (base material of medical implants). Finally some other options can be a good research area for finding new methods and helping to obtain better result in this method; also it can help us to figure out the behavior of HAp water mixture and effects of the other materials and temperatures on it during the process. In example the temperature affect on the color of adhered HAp on the inner layer of the ceramic mould. Also one of the problems that occurred in this project was the measuring the viscosity of HAp-water mixture, because of its creamy characteristic. As it was mentioned before the brittleness of HAp layer was also a difficulty to obtain accurate results during the test for example measuring the thickness of HAp layer was to hard and using usual measuring methods was impossible thus finding new methods and new materials to decrease the brittleness of HAp layer can be a good subject. 66 References Abrams L, Bajpai PK. 1994. Hydroxyapatite ceramics for continuous delivery of heparin. Biomed. Sci. Instrumen. 30:169–174 Bajpai PK and Fuchs CM. 1985. Development of a Hydroxyapatite Bone Grout. In: Proceedings of the First Annual Scientific Session of the Academy of Surgical Research. San Antonio, Texas. C. W. Hall, Ed. p. 50–54. Pergamon Press, New York, NY.] Bajpai PK. 1992. Ceramics: A Novel Device for Sustained Long Term Delivery of Drugs. In: Bioceramics Volume 3. JA Hulbert and SF Hulbert, Eds. p. 87–99. RoseHulman Institute of Technology, TerraHaute, IN] Bajpai PK. 1994. Ceramic Drug Delivery Systems. In: Biomedical Materials Research in The Far East (I). Xingdong Zhang and Yoshito Ikada, Eds. p. 41–42, 1994. Kobunshi Kankokai Inc., Kyoto, Japan.Parker and Bajpai, 1993] Bajpai PK. 1992. Ceramics: A Novel Device for Sustained Long Term Delivery of Drugs. In: Bioceramics Volume 3. JA Hulbert and SF Hulbert, Eds. p. 87–99. RoseHulman Institute of Technology, Terra Haute, IN] Bunyamin Aksakal C. Hanyaloglu, Bioceramic dip-coating on Ti–6Al–4V and 316L SS implant materials, Sci:Mater Med 2007] 67 Cition. American Ceramic Society 26 th Annual Meeting Conference Proceedings. 2003] Gutwein, LG.; Webster, TJ. Affects of alumina and titania nanoparticulates on bone cell foundation] D.A. Cortes, A.A. Nogiwa , J.M. Almanza , S. Ortega., coating onMg-PSZ/Al2O3 composites. Effect of the immersion method, Materials Letters 59 1352– 1355 (2005)] De la Isla A, Brostow W, Bujard B, Estevez M, Rodriguez JR, Vargas S, Castano VM. Nanohybrid scratch resistant coating for teeth and bone viscoelasticity manifested in tribology. Mat Resr Innovat. 2003;7:110–114.]. Feenstra L and de Groot K. 1983. Medical Use of Calcium Phosphate Ceramics. In: Bioceramics of calcium phosphate. K de Groot, Ed. p. 131–141, CRC Press, Boca Raton, FL] Gutwein, LG.; Webster, TJ. Affects of alumina and titania nanoparticulates on bone cell function. American Ceramic Society 26 th Annual Meeting Conference Proceedings. 2003] Hench LL. 1993. Bioceramics: From concept to clinic. American Ceramic Society Bulletin, 72:93–98]. Hench LL. 1991. Bioceramics: From concept to clinic. J. Am. Ceram. Soc. 74:1487– 1510] Hentrich RL Jr, Graves GA Jr, Stein HG, and Bajpai PK. 1971. An evaluation of inert and resorbable ceramics for future clinical applications. J. Biomed. Mater. Res. 5:25–51] 68 H.H. Rodrı´guez H.H. Rodrı´guez, G. Vargas, D.A. Corte´s., Electrophoretic deposition of bioactive wollastonite and porcelain–wollastonite coatings on 316L stainless steel, Ceramics International (2007)] Jarcho M, Salsbury RL, Thomas MB, and Doremus RH. 1979. Synthesis and fabrication of β-tricalcium phosphate (whitlockite) ceramics for potential prosthetic applications. J. of Mater. Science. 14:142–150] J.C. Escobedo, et al; Hydroxyapatite coating on a cobalt base alloy by investment casting Scripta Materialia 54 (2006) 1611–1615] Jie Weng , Formation and characteristics of the apatite layer on plasma-sprayed hydroxyapatite coatings in simulated body fluid, Biomoterials vol.18 (1997)] Kingery WD, Bowen HK, and Uhlmann DR. 1976. Introduction to Ceramics, 2nd ed., p.368, J. Wiley, New York] Ma J, Wong H, Kong LB, Peng KW. Biomimetic processing of nanocrystallite bioactive apatite coating on titanium. Nanotechnology. 2003;14:619– 623. doi: 10.1088/0957-4484/14/6/310.] N. Ramesh Babu et al. Trends Biomater. Artif. Organs. Vol. 17(2) pp 43-47 (2004)] Park JB and Lakes RS. 1992. Biomaterials—An Introduction, 2nd ed., Plenum Press, New York]. Peltier LF. 1961, The use of plaster of Paris to fill defects in bone. Clin. Orthop, 21:1–29] 69 Piattelli A and Trisi P. 1994. A light and laser scanning microscopy study of bone/hydroxyapatite-coated titanium implants interface: Histochemical evidence of unmineralized material in humans. J. Biomed. Mater. Res. 28:529–536] R.M. Trommer, Alternative technique for hydroxyapatite coatings, Flame Assisted Chemical Vapor Deposition (FACVD)Surface & Coatings Technology 201 (2007)] TuantuanliI, Junhee Lee ,Takayuki Kobayashi, Hideki Aoki, Hydroxyapatite coating by dipping method, and bone bonding strength, Journal of material science, Materilas in Medicine, 7 (1996) 355 357]