Prof.Dr.Nadeem Mohammed Taher Collage of Nursing
advertisement
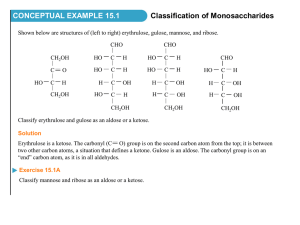
Prof.Dr.Nadeem Mohammed Taher Collage of Nursing Biochemistry:-this term was introduced by Carl Neuberg In 1903 (chemical language of life basic to understanding Biological &medical sciences Q-Is it essential for the Nurse to study biochemistry? 1- To understanding the basic function of human body 2- To give information regarding the function of the cell 3- To know how the food is digested, absorbed and used for body building 4- To understand how the body gets energy for day to day 5- To understand the interrelation between various metabolic processes in the body Chapter :1 carbohydrates Carbohydrate include a large group of compounds commonly known as starches or plants and animals Plants -------Glucose(dextrose) Fructose(levelose) Sucrose(sugar cane) Maltose starch sugars which are widely distributed in animals ----------glucose(blood sugar) glycogen(liver & muscle) lactose(sugar milk) galactose(glycolipids) Carbohydrate define as polyhydroxy aldehydes or polyhydroxy ketones or substances that Yild such compounds on hydrolysis H | C=O | H - C- OH | HO-C-H | H - C- OH | H- C-OH | CH2OH GLUGOSE *carbohydrate CH2OH | C=O | HO- C-H | H-C-OH | H-C-OH | CH2OH FRUCTOSE formula Cn(H2O)n ----------------- hydrate of carbon BIOLOGIC IMPORTANCE --------------------------------------------------------------- 1- source of energy in the body 2- proteins sparing action of carbohydrate 3- source of energy for CNS is glucose----1-hypoglycaemia--(damage of the brain) 2-hyperglycaemia 4- add flavour and variety to diet Q- what are the medical importance of carbohydrate? Types of carbohydrates Classifications based on number of sugar units in total chain. Monosaccharides - single sugar unit Disaccharides - two sugar units Oligosaccharides - 2 to 10 sugar units Polysaccharides - more than 10 units Chaining relies on ‘bridging’ of oxygen atoms glycoside bonds CLASSIFICATION ------------------------------------------------ 1- monosaccharide (simple sugar) (CH2O)n n=3 or more This group subdivided into (a) Depending upon the function group H CH2OH | | C=O C=O | | H- C-OH HO-C-H | | H-C-OH H-C-OH | | H-C-OH H-C-OH | | CH2OH CH2OH Aldose Ketose (B) Depending upon the number of carbon atoms General formula Aldosugar ketosugar -------------------------------------------------------------------------Triose(C3H6O3) Glyceraldehyde Dihydroxyaceton Tetrose(C4H8O4) Erythrose Erythrulose Pentose(C5H10O5 ) Ribose Ribulose Hexose(C6H12O6) Glucose Fructose H | C=O | H- C-OH | CH2OH triose H | C=O | H- C-OH | H-C-OH | CH2OH tetrose H | C=O | H- C-OH | H- C-OH | H- C-OH | CH2OH pentose Can be either aldose or ketose sugar. H- HHH- H | C=O | C-OH | C-OH | C-OH | C-OH | CH2OH hexose Examples H | C=O | H-C-OH | CH2OH D-glyceraldehyde triose aldose aldotriose sugar CH2OH | C=O | HO- C-H | H- C-OH | H- C-OH | CH2OH D-fructose hexose ketose ketohexose sugar Examples H | C=O | H- C-OH | H- C-OH | H- C-OH | CH2OH D-ribose pentose, aldose aldopentose sugar HHHOHO- H | C=O | C-OH | C-OH | C-H | C-H | CH2OH L-mannose hexose, aldose aldohexose sugar Q-draw the structure of the carbohydrate? 1- L-glucose 2- L-glyceraldehyde 3- Ribulose 4- D-galactose 5- arabinose 2- Disaccharides: these sugars are yield 2 molecules of the same or different molecules of monosaccharide on hydrolysis Lactose EXAMPLE Milk sugar - dimer of -D-galactose and either the or - D-glucose. -Lactose CH2OH O OH H H O OH H OH H H H H OH OH H H CH2OH O OHH -D-galactose (1 4) linkage, disaccharide. -D-glucose Sucrose CH2OH Table sugar - most common sugar in all plants. Sugar cane and beet, are up to 20% by mass sucrose. Disaccharide of glucose and fructose. O H H OH H H OH OH O CH2OH O H (1 H OH H 2) linkage OH H CH2OH Maltose Malt sugar. Not common in nature except in germinating grains. CH2 OH CH2 OH H H OH O H H H O H OH O H H OH H OH -D-glucose OH H OH -D-glucose -D-glucose and -D-glucose, (1 4) linkage. 3- Oligosaccharides: these sugars are yield 2-10 molecules of monosaccharide on hydrolysis EXAMPLE (Raffinose----------(glucose +fructose + galactose) 4- Polysaccharides: these compounds yield many molecules of monosaccharide on hydrolysis General formula (C6H10O5)n This group are subdivided into two groups (a) Homopolysaccharide:polymer of the same monosaccharide units e.g starch , glycogen , inulin , cellulose Starch Energy storage used by plants Long repeating chain of -D-glucose Chains up to 4000 units Amylose major form of starch straight chain Amylopectin branched structure Amylose starch Straight chain that forms coils (1 common type of starch. O H H OH H O H H OH H H H OH H H O O O H OH H H O O O O H H OH O O O O O O O O H O OH O O O H O OH O O H O OH O O H O H CH2OH CH2OH CH2OH CH2OH 4) linkage. Most O O O O Amylopectin starch Branched structure due to crosslinks. O H H OH H O H H OH H H H O H H OH H OH H CH2OH H OH H O H H OH H O H H (1 H O CH2OH H OH H O H H OH H OH CH2 OH H O H H OH H H 6) linkage at crosslink OH H H O O O OH H O O H H O CH2OH H OH O H H OH O H CH2OH CH2OH CH2OH CH2OH H OH Glycogen • Energy storage of animals. • Stored in liver and muscles as granules. • Similar to amylopectin. (1 O O O O 6) linkage at crosslink O O O O O O O O O O Cellulose • Most abundant polysaccharide. • (1 4) glycosidic linkages. • Result in long fibers - for plant structure. CH2OH CH2OH CH2OH CH2OH CH2OH O H H OH O H OH H OH O H H OH H OH O H H OH H OH O H H OH H OH O H H H H H H H H O H H O O H O H OH Inulin Inulin = polymer of fructose (B) Heteropolysaccharides :polymer of different monosaccharide units or their derivatives . Example 1- hyaluronic acid (glucuronic acid + N-acetyl hexosamine ) 2- mucopolysaccharide ( hexose + hexosamine) 3- chondroitin ( glucuronic acid+ N- actylgalactosamine + sulphate ) 4- heparin (glucuronic acid + glucosamine N- sulphate + sulphate ester) Thank you