18.4/18.5

18.4/18.5
ACID-BASE TITRATIONS AND INDICATORS
Titration Introduction:
A titration is a controlled addition and measurement of the amount of a solution of _________concentration (mol dm
-3
) required to react completely with a measured amount of a solution of unknown concentration (mol dm
-3
).
In a titration we are trying to reach the _______________ point. This is the point at which the two solutions are present in exactly the same amounts. (i.e.moles)
In a titration we use
___________solutions to show us when the _____-point has been reached. The end point is the point at which the indicator changes ______. It is important that we pick an indicator that will change color in a range that includes the pH of the equivalence point. (More on this later.)
SUMMARY OF SOME IMPORTANT TITRATION TERMS:
*titration
the precise addition of solution in a ________________ into a ______________ volume of a sample solution
*titrant
the solution in the ________________ during a titration
the concentration is of the titrant must be _____________
The titrant is added to the ____________ until it is __________________ . The volume of titrant needed to neutralize the sample is recorded and used to calculate the __________________ of the sample.
*sample
the solution being analyzed in a titration
the ______________ of the sample is known; the __________________is unknown
*endpoint the point in a titration at which a sharp change in a measurable and characteristic property occurs [e.g. a ___________ change in an acid-base ______________ / e.g. phenolphthalein indicator is ___________in acid solution and _____ in basic solution – when it turns pink the __________ has been reached] *Indicators are usually __________ acids themselves.
* equivalence point
the point in the titration where the amount (#of moles) of
_________ ___added reacts with the ___________ amount of reactant in the ___________(
*Remember
When performing a titration it is necessary to choose an indicator that has an endpoint that is approximately equal to the equivalence point.
1
Titration Type #1
STRONG ACID WITH STRONG BASE
Ex. 1: T he concentration of a hydrochloric acid sample needs to be determined. A 10.0 cm 3 sample of the acid is titrated with 0.10 mol dm -3 sodium hydroxide. 13.5 cm 3 of titrant (i.e.
NaOH) are added before the endpoint is reached. Determine the concentration of the acid.
*Write a balanced chemical equation and then determine whether the products of the neutralization reaction affect the pH?…do they react with H
2
O(i.e. hydrolyze)?
*At EQUIVALENCE POINT: pH = _______ (i.e. the titration is complete when the pH of the resulting solution equals ______. Therefore, the chosen indicator should turn colour (i.e. reach its ENDPOINT ) at a pH of _______ .
Ex. 2 : A 25.0 cm 3 sample of calcium hydroxide is titrated with 0.22 mol dm -3 nitric acid. It takes
18.3 cm 3 of titrant to neutralize the sample. Determine the concentration of the sample.
*Write a balanced chemical equation and then determine whether the products of the
neutralization reaction affect the pH? *At EQUIVALENCE POINT: pH = _______
Titration Type #2
WEAK ACID WITH STRONG BASE
Ex.: It takes 42.5 cm 3 of 1.02 mol dm -3 NaOH solution to neutralize the ethanoic acid in a
50.0 cm 3 sample of vinegar. What is the concentration of the acetic acid in the vinegar?
*Write a balanced chemical equation and then determine whether the products of the
neutralization reaction affect the pH? *At EQUIVALENCE POINT: pH ___ 7
2
Titration Type #3
STRONG ACID WITH WEAK BASE
Ex.
Write the equation for a neutralization reaction involving ammonia and hydrochloric acid.
How do the products of the neutralization reaction affect the pH?
*At EQUIVALENCE POINT: pH ___ 7
Titration Type 4: Weak Acid with Weak Base (e.g. ethanoic acid and ammonia)
*Only small change in pH at the equivalence point, making it difficult to detect. This type of titration is very rarely (if ever) performed since titrating with a strong acid or base is always an option.
Summary:
“Type” of Titration
Entity determining pH at equivalence point pH at equivalence point
Strong Acid with Strong Base e.g.
Weak Acid with Strong Base e.g.
Strong Acid with Weak Base e.g.
Weak Acid with Weak Base e.g.
*Titration Calculations (Text Homework):
Read pp. 29-31; Answer pp. 31-32 #1-10
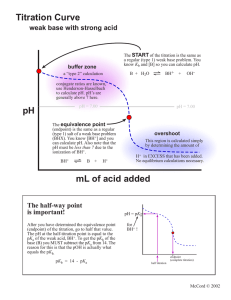
TITRATION CURVES:
Strong Acid / __________ ______________/_____________
3
_______________/_______________
*How can the pK a
of the acid be determined from the graph on the left?
*Hint: Recall the Henderson-Haselbach equation…what is significant about the
“half-way to equivalence point”?
*Label the “buffer region” on the graph.
*How could the pK b be determined from the previous graph (i.e. the “weak base/strong acid graph)
*CHOOSING AND APPROPRIATE INDICATOR:
*At the equivalence point: pH =
*That is: determine pH at equivalence point, then choose an indicator with a pK a
approximately equal to that pH.
(See table 16 in data booklet.)
Indicators (Review)
An indicator is a ________ acid (or base) that has different colours in different pHs. An indicator is represented generically by HIn – it is a special case of HA, the weak acid.
HIn
(aq)
H +(aq) + In -(aq)
(colour 1) (colour 2)
Adding H + will shift the equilibrium to the left and colour 1. Whereas addition of OH will remove H + and shift the equilibrium to the right and colour 2.
The _______ of the indicator gives us the pH where the colour change is centered.
4