Problem set 8 Chemical Bonding
advertisement

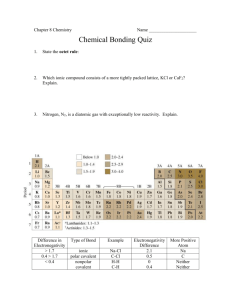
Problem set 8 Chemical Bonding 1. Classify each bond as nonpolar covalent, polar covalent, or ionic (use the electronegativity values on page 341 of your textbook): a) Al – F b) Se – Br c) Si – H d) Ca – Cl 2. Arrange the elements in each set in order of decreasing electronegativity (by using only the Periodic Table): a) O, C, N, F b) Br, F, Cl, I c) P, S, Al, Cl d) K, Li, Na, Cs 3. Draw a Lewis structure for each covalent compound: a) CCl4 b) C2H6 c) OF2 d) H2O2 4. Which of the following molecules are polar? Why? a) NH3 b) CH2F2 c) CCl4 d) CO2 5. Predict the shape of each molecule and bond angle by using VSEPR model: a) NO2b) SiH4 c) OF2 d) C2H4 6. In AlCl3, the electron configuration of aluminium ion is similar to which noble gases? What about chloride ion? Dr. Behrang Madani Chemistry 100 IVC 7. Find the charge of each element for these ionic compounds: a) CaO b) PbCl4 c) HgS d) Cu2O e) KBr f) Fe2O3 8. Use the following ions to write the chemical formula for each ionic compound: a) Ga3+ O2- b) Ag+ I- c) Be2+ S2- d) Ga3+ Br- 9. For problem 8, write the electron configuration for each ion. They are similar to which noble gases? 10. Draw a Lewis structure for each ion: a) CO32b) NH4+ c) H3O+ d) SO4211. Use the electron configuration of calcium and oxygen and then Ca2+ and O2- to show how an ionic bond is produced between them. Dr. Behrang Madani Chemistry 100 IVC