Chapters 2-4 review
advertisement
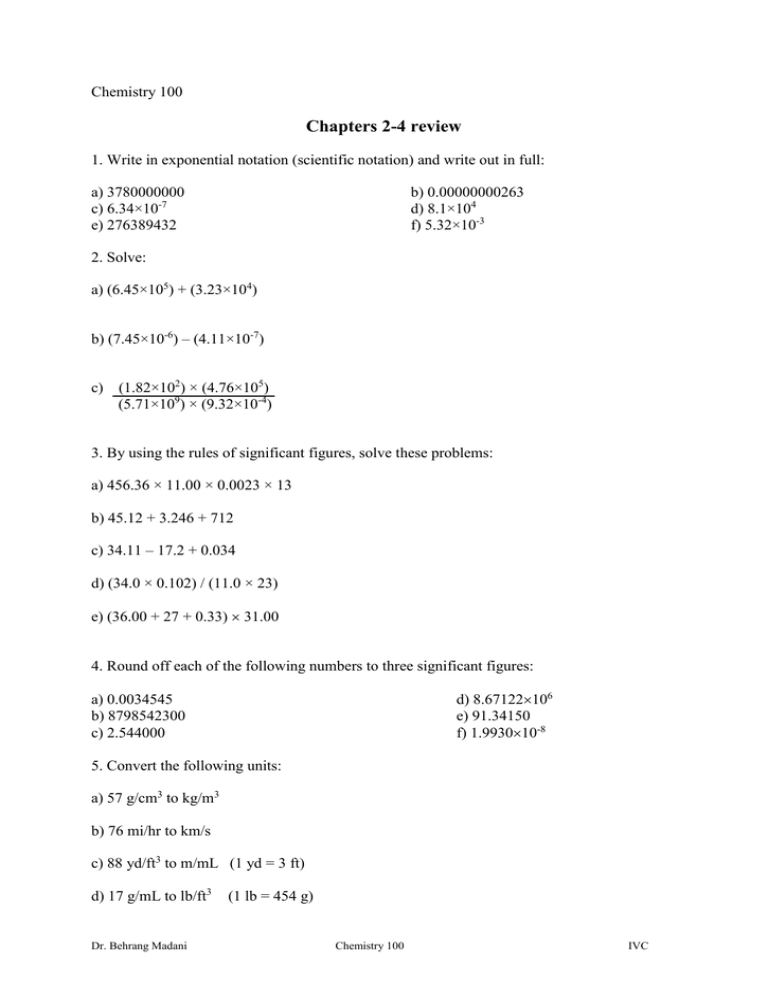
Chemistry 100 Chapters 2-4 review 1. Write in exponential notation (scientific notation) and write out in full: a) 3780000000 c) 6.34×10-7 e) 276389432 b) 0.00000000263 d) 8.1×104 f) 5.32×10-3 2. Solve: a) (6.45×105) + (3.23×104) b) (7.45×10-6) – (4.11×10-7) c) (1.82×102) × (4.76×105) (5.71×109) × (9.32×10-4) 3. By using the rules of significant figures, solve these problems: a) 456.36 × 11.00 × 0.0023 × 13 b) 45.12 + 3.246 + 712 c) 34.11 – 17.2 + 0.034 d) (34.0 × 0.102) / (11.0 × 23) e) (36.00 + 27 + 0.33) 31.00 4. Round off each of the following numbers to three significant figures: d) 8.67122106 e) 91.34150 f) 1.993010-8 a) 0.0034545 b) 8798542300 c) 2.544000 5. Convert the following units: a) 57 g/cm3 to kg/m3 b) 76 mi/hr to km/s c) 88 yd/ft3 to m/mL (1 yd = 3 ft) d) 17 g/mL to lb/ft3 Dr. Behrang Madani (1 lb = 454 g) Chemistry 100 IVC 6. Iron has a density of 7.874 g/cm3. What is the mass of a rectangular block of iron in kilogram with dimensions of 0.098 ft by 0.004 m by 1.968 in? (1ft = 12 in and 1in = 2.54 cm). Find the specific gravity of iron. 7. Copper has density 8.96 g/cm3. If 52.4 g of copper is added to 75.0 mL of water in a graduated cylinder, to what volume reading will the water level in the cylinder rise? 8. What Celsius temperature is the same as 77.0°F? What is the temperature on Kelvin scale? 9. Use an effective physical method to separate the following mixtures: a) Sand and water b) Alcohol and water c) Moisture in oil d) Dust and air 10. What is the difference between physical properties of gas, liquid, and solid? 11. What is the atomic number and mass number of these elements? a) 24 protons, 24 electrons, 26 neutrons b) 106 protons, 106 electrons, 150 neutrons Dr. Behrang Madani Chemistry 100 IVC 12. How many protons, electrons, and neutrons are contained in the nucleus of each of the following atoms? a) 39 24 Cr b) 84 34 Se c) 75 33 As d) 41 19 K 13. The natural abundance of an element is as follows: 19.9% (10.013 amu) for the first isotope and 80.1% (11.009 amu) for the second isotope. Calculate the atomic weight and find the name of this element by using the Periodic Table. 14. Describe the physical properties of the metals and nonmetals: 15. Which element in each pair is more metallic? a) C or N b) Mg or Ca c) Cs or Ba d) S or Cl 16. Verify the charge of these ions and correct them: I2+ Mg3+ Dr. Behrang Madani K3+ P2- Chemistry 100 Ne- H2+ IVC











