Ionic and Covalent Bonding Ionic bonding: Covalent bonding:
advertisement
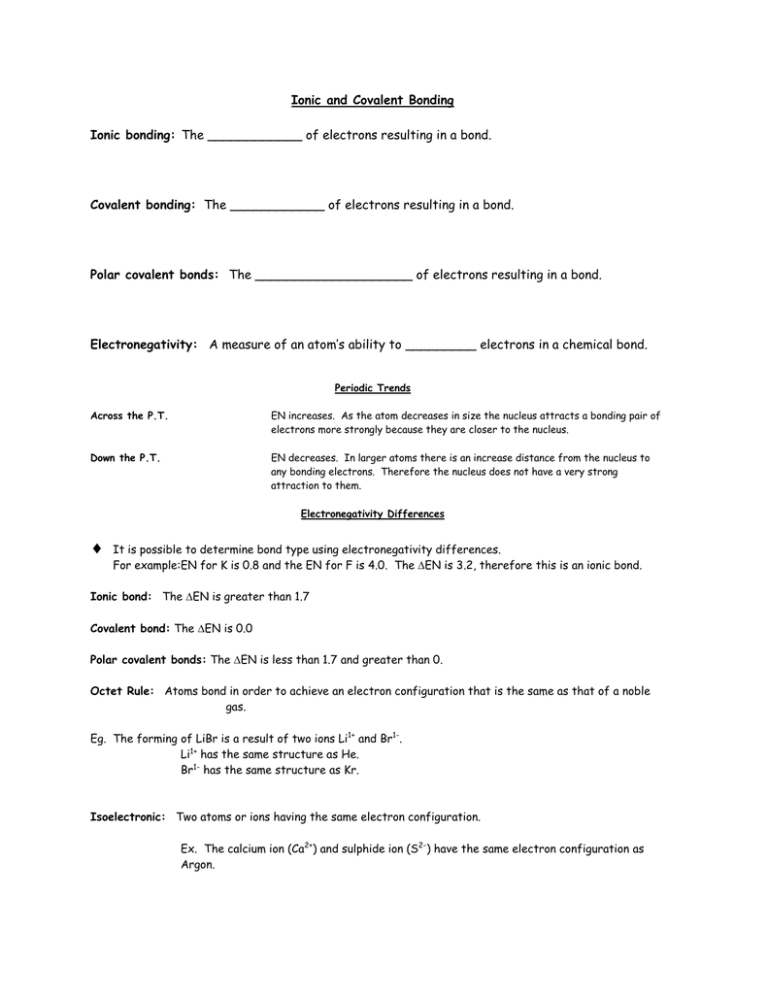
Ionic and Covalent Bonding Ionic bonding: The ____________ of electrons resulting in a bond. Covalent bonding: The ____________ of electrons resulting in a bond. Polar covalent bonds: The ____________________ of electrons resulting in a bond. Electronegativity: A measure of an atom’s ability to _________ electrons in a chemical bond. Periodic Trends Across the P.T. EN increases. As the atom decreases in size the nucleus attracts a bonding pair of electrons more strongly because they are closer to the nucleus. Down the P.T. EN decreases. In larger atoms there is an increase distance from the nucleus to any bonding electrons. Therefore the nucleus does not have a very strong attraction to them. Electronegativity Differences It is possible to determine bond type using electronegativity differences. For example:EN for K is 0.8 and the EN for F is 4.0. The EN is 3.2, therefore this is an ionic bond. Ionic bond: The EN is greater than 1.7 Covalent bond: The EN is 0.0 Polar covalent bonds: The EN is less than 1.7 and greater than 0. Octet Rule: Atoms bond in order to achieve an electron configuration that is the same as that of a noble gas. Eg. The forming of LiBr is a result of two ions Li1+ and Br1-. Li1+ has the same structure as He. Br1- has the same structure as Kr. Isoelectronic: Two atoms or ions having the same electron configuration. Ex. The calcium ion (Ca2+) and sulphide ion (S2-) have the same electron configuration as Argon.




