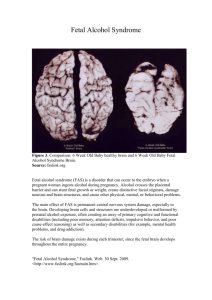
ARTICLE IN PRESS The importance of dawarenessT for understanding fetal pain
advertisement

DTD 5 ARTICLE IN PRESS BRESR-100324; No. of pages: 17; 4C: 7 Brain Research Reviews xx (2005) xxx – xxx www.elsevier.com/locate/brainresrev Review The importance of dawarenessT for understanding fetal pain David J. Mellora,T, Tamara J. Diescha, Alistair J. Gunnb, Laura Bennetb a Riddet Centre and Institute of Food, Nutrition and Human Health, College of Sciences, Massey University, Palmerston North, New Zealand b Department of Physiology, University of Auckland, Private Bag 92019, Auckland, New Zealand Accepted 12 January 2005 Abstract Our understanding of when the fetus can experience pain has been largely shaped by neuroanatomy. However, completion of the cortical nociceptive connections just after mid-gestation is only one part of the story. In addition to critically reviewing evidence for whether the fetus is ever awake or aware, and thus able to truly experience pain, we examine the role of endogenous neuroinhibitors, such as adenosine and pregnanolone, produced within the feto-placental unit that contribute to fetal sleep states, and thus mediate suppression of fetal awareness. The uncritical view that the nature of presumed fetal pain perception can be assessed by reference to the prematurely born infant is challenged. Rigorously controlled studies of invasive procedures and analgesia in the fetus are required to clarify the impact of fetal nociception on postnatal pain sensitivity and neural development, and the potential benefits or harm of using analgesia in this unique setting. D 2005 Elsevier B.V. All rights reserved. Theme: Disorders of the nervous system Topic: Pain Keywords: Fetus; Nociception Contents 1. Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . 2. Fetal responses to nociceptive stimuli . . . . . . . . . . . . . 2.1. Neuroanatomical maturation . . . . . . . . . . . . . . . . 2.2. The question of cortical awareness . . . . . . . . . . . . 2.3. Is the fetus ever awake? . . . . . . . . . . . . . . . . . . 2.3.1. Characterization of wakefulness . . . . . . . . . . 2.3.2. Sleep-like states in the fetus . . . . . . . . . . . . 2.3.3. Fetal arousal or sleep state transitions? . . . . . . . 2.3.4. Evidence that the fetus remains asleep . . . . . . . 2.4. Sleep transitions—an indeterminate state of sleep . . . . . 2.5. Can the fetus be woken up? . . . . . . . . . . . . . . . . 2.6. Can the fetus dream? . . . . . . . . . . . . . . . . . . . 2.7. Summary of bfetal wakefulnessQ. . . . . . . . . . . . . . 3. Suppressors of fetal behavior and cortical activity . . . . . . . 3.1. Adenosine and oxygen status . . . . . . . . . . . . . . . 3.2. Neuroactive steroids—allopregnanolone and pregnanolone T Corresponding author. E-mail address: D.J.Mellor@massey.ac.nz (D.J. Mellor). 0165-0173/$ - see front matter D 2005 Elsevier B.V. All rights reserved. doi:10.1016/j.brainresrev.2005.01.006 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 ARTICLE IN PRESS 2 D.J. Mellor et al. / Brain Research Reviews xx (2005) xxx–xxx 3.3. Prostaglandin D2 . . . . . . . . . . . . . . 3.4. Placental peptide inhibitor and other factors 3.5. Thermal status—warmth . . . . . . . . . . 3.6. Tactile stimulation. . . . . . . . . . . . . . 3.7. Summary of endogenous inhibition . . . . . 4. Long-term sequelae . . . . . . . . . . . . . . . 5. Summary and conclusions . . . . . . . . . . . . Acknowledgments . . . . . . . . . . . . . . . . . References . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1. Introduction Whether the fetus can truly experience pain, at least in some way analogous to how adults emotionally understand pain, has been debated extensively over recent years and is of importance given continuing advances in fetal surgical and diagnostic procedures [43]. This question has considerable implications for the management of invasive fetal procedures [16,78,94], particularly as fetal analgesic and anaesthetic treatment is complex [195] and not without risk for the fetus [171]. Prevention and treatment of pain are basic human rights, regardless of age, and if fetal interventions are to progress, then a greater understanding of nociception and stress responses is required [236]. The timing of the neuroanatomical maturation of the nociceptive system is now well understood, and the final critical cortico-thalamic connections appear to be present by 24–28 weeks of gestation [25,40,56,78,136,236]. This suggests that the fetus could potentially be able to feel pain by the third trimester, at least in a rudimentary fashion. This concept is said to be supported by studies which show that nociceptive stimuli elicit physiological stress-like responses in the human fetus in utero [210]. However, physiological processing of a nociceptive stimulus and perceiving a nociceptive stimulus as painful are not the same. There are both a physiological and an emotional or cognitive aspect to pain perception, and indeed a significant element of learning [56]. Certainly, processing can be independent of perception, as is demonstrated during surgery under general anesthesia, for example, where nociceptive stimuli can still elicit subcortically mediated physiological stress responses despite unconsciousness [57,85,140]. Thus, to emotionally experience pain, we must be cognitively aware of the stimulus (a cortical process), and this in turn requires that we must be conscious [25,40,56]. The key question then is not about the anatomic completion or functionality of nociceptive pathways in utero, but whether the fetus is ever conscious and thus aware. In general, discussion of fetal pain perception tends to treat the fetus as an unborn newborn; i.e., that responses of the newborn represent an adequate surrogate for the fetus. The assumption is thus made that if the newborn (including the preterm newborn) can experience wakefulness (and there- . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 0 0 0 0 0 0 0 0 0 fore consciousness), and apparently feels pain, then so too must the age-equivalent fetus. Furthermore, evidence for fetal wakefulness (and again therefore consciousness) has been based on how certain fetal responses bresembleQ newborn sleep–wake behaviors, rather than a true determination of fetal wakefulness per se. Given the complexities of studying the fetus, extrapolation from or to the newborn state is understandable. Systematic studies of fetal neurological function suggest, however, that there are major differences in the in utero environment and fetal neural state that make it likely that this assumption is substantially incorrect. This has important implications for our understanding of fetal pain perception. The current review critically evaluates the hypothesis that unlike the newborn, the fetus is actively maintained asleep (and unconscious) throughout gestation and cannot be woken by nociceptive stimuli. The evidence is examined with reference to fetal sleep–wake states, the role of corticothalamic gating in cortical arousal during sleep, and the unique contribution that certain inhibitory neuromodulators make in utero to cortical suppression. Finally, we briefly discuss the validity of the hypothesis that suggests that the nociceptive input may have long-lasting deleterious effects regardless of whether the fetus is asleep or not. 2. Fetal responses to nociceptive stimuli 2.1. Neuroanatomical maturation The processing of nociceptive stimuli requires peripheral sensory receptors, afferent and efferent sensory and motor pathways, and subcortical and cortical neural integration of the related impulse traffic [8]. The development of nociceptive pathways has been extensively reviewed by others and is not the subject of this review [13,15,56,64,66, 118,174,230,236]. In brief, however, it is generally agreed that an integrated pathway exists by 24–28 weeks of gestation and that it includes the critical cortico-thalamic connections deemed to be essential for the experience of pain [78,136]. The thalamus is an obligatory station through which nearly all sensory information must pass before reaching the cerebral cortex. One major function of the thalamus is the selective control of the flow of sensory- ARTICLE IN PRESS D.J. Mellor et al. / Brain Research Reviews xx (2005) xxx–xxx motor information to the cerebral cortex during different states of the sleep–wake cycle and arousal, modulated by inputs from the brainstem, hypothalamus, and cerebral cortex [146]. Several commentaries suggest that once the nociceptive pathway is complete the fetus may experience pain and that various behavioral and physiological responses may reveal fetal awareness or subjective consciousness of pain [25,78,210]. For instance, the human fetus responds to intrahepatic needling (versus umbilical cord sampling) by moving away and with an increase in the levels of circulating stress hormones such as cortisol, corticotropin releasing hormone (CRH), catecholamines, and beta-endorphins [71,72,74,75]. These responses are independent of maternal responses [72,74]. Moreover, fetal intrahepatic sampling leads to a redistribution of the combined ventricular output away from peripheral organs such as the gut and kidneys towards central organs such as the heart and brain [211,224], and scalp sampling increases heart rate in some fetuses [215]. 2.2. The question of cortical awareness It is noteworthy, however, that these responses are elicited at the subcortical and brainstem level and do not require cortical input [78,136,243]. Thus, they cannot be said to represent evidence for cortical awareness. For example, very similar cardiovascular redistribution responses are seen during severe experimental fetal hypoxemia as part of a coordinated fetal defense, and occur even when neural activity is essentially isoelectric; i.e., when fetal brain activity is profoundly suppressed [30,76,107,112,115]. Consistent with this, stress hormones are significantly elevated during surgical procedures carried out under general anesthesia [57,85,140] and in brain dead patients during organ harvesting [68,137]. Indeed, even an anencephalic fetus responds to somatic stimulation by withdrawal, demonstrating that these reflexes are mediated entirely at a subcortical level [233]. Similarly, patients in vegetative states are thought to be incapable of conscious experience themselves, even though they are able to be aroused [242], and in such patients flexor withdrawal and other reflexes, which are mediated through the thalamus, are preserved [179]. In the condition of sleepwalking, motor arousal can clearly occur, but while sleep is disturbed it is not to the extent of full awakening [5]. Similarly, in the neonate, arousal-eliciting stimuli can also cause respiratory and motor responses without necessarily leading to cortical arousal [149]. Likewise, moderate pain can elicit physiological and behavioral responses during sleep in adults without the subject arousing or waking [69]. These variable cortical states demonstrate that hormonal and arousal responses to noxious stimuli can be elicited independent of cortical awareness. This, however, does not mean that fetal awareness does not occur. The question is then, is the fetus ever conscious? 3 2.3. Is the fetus ever awake? 2.3.1. Characterization of wakefulness The existence of consciousness is, of course, difficult to establish in the fetus, but wakefulness might be used as a provisional index, provided it is borne in mind that wakefulness and consciousness or awareness are quite different states [63,243]. Wakefulness is a state of brainstem and thalamic activity; a state of non-sleep-arousal, whereas consciousness requires cortical processing [63,243]. In order for consciousness to occur, all the incoming information from the internal and external environment must be available to all parts of the cortex at the same time [63]. Moreover, sleep is an arousable state of unconsciousness [243]. Thus, it is possible to be awake and not conscious (as in some persistent vegetative states and in conditions of blindsight [242]) and to be awake and conscious, but it is not possible to be asleep and conscious. It follows that if the fetus was shown never to be awake, it would always be unconscious. Whereas if wakefulness was evident, even transiently, it would indicate the possibility that the fetus may achieve states of consciousness, but such wakefulness would not represent unequivocal evidence for consciousness. 2.3.2. Sleep-like states in the fetus The development of sleep-like states and behavior in the fetus has been well defined, both clinically and experimentally [17,50,110,143,168,170,186,219]. Of brief note regarding experimental models, the experimental preparation most often used to systematically study the developing fetus is that of the chronically instrumented sheep fetus. This approach permits the physiological and neurological monitoring of the fetus in utero well after surgical manipulation and without the confounding effects of anesthesia. Further, physiological responses in the fetal sheep are consistent with those observed in the human. From mid-gestation, the fetal electroencephalogram (EEG) begins to evolve from the discontinuous patterns of tracé alternant, which is associated with the constant simultaneous expression of numerous fetal behaviors (Fig. 1), into coherent, discrete states which are suggestive of sleep and are referred to as sleep states [50,170,183]. By late gestation, as is seen postnatally, there are two well-defined sleep states, that of rapid-eye-movement (REM) or active sleep, characterized by low-voltage high-frequency EEG activity, and non-rapid-eye-movement (NREM) or quiet sleep, characterized by high-voltage low-frequency EEG activity (Fig. 2) [188]. In late gestation, these states account for 95% of fetal EEG activity, they are episodic and during each state the fetus tends to exhibit specific behaviors. Generally breathing, swallowing, licking, and eye movements, and atonia occur in REM sleep, whereas apnea, absence of eye movements, and tonic muscle activity occur in NREM sleep [50,188,219]. These observations demonstrate that sleep, or unconsciousness, is the dominant fetal state for at least 95% of the ARTICLE IN PRESS 4 D.J. Mellor et al. / Brain Research Reviews xx (2005) xxx–xxx Fig. 1. Example of a chart recorder tracing from a sheep fetus at 91 days of gestation (0.6 gestation, term = 147 days). This stage of maturity corresponds with the human brain at around 26–28 weeks of gestation. The trace shows changes in fetal electroencephalogram (EEG), electrooculogram (EOG), nuchal electromyogram (EMG), and tracheal pressure measurements. Note the lack of clearly defined sleep state and the near-continuous presence of eye, body, and breathing movements which typify behavior in the immature fetus. The first half of the trace is run at a higher speed to show that a variety of fetal activities frequently occur coincidentally. Bar A = 10 s, bar B = 10 min. time. The obvious and critical question is what state lategestation fetuses are in during the 5% of the time that they are apparently not in REM or NREM sleep? Are the fetuses awake or merely in transition between the two sleep states, and how is an awake state defined? Prechtl described the categorization of state-related behavior as follows: bby the term state, one tries to describe constellations of certain functional patterns and physiological variables that may be relatively stable and that seem to repeat themselvesQ [182]. Postnatally, wakefulness is defined clinically by reference to opening and moving of the eyes and purposeful movements of the head; there is high muscle tone superimposed on general body movements; increased and irregular heart rate and respiration; and a low-voltage, irregular, mixed pattern of EEG with frequent movement artefacts [92,157]. 2.3.3. Fetal arousal or sleep state transitions? Using this definition of neonatal wakefulness, two barousalQ states have been suggested to occur in the human fetus [54,73,168]. The fetus is said to be actively awake (in state F4) when vigorous, continual activity, including trunk rotations, and eye movements are present and its heart rate is unstable with long accelerations [168]. Fetal bwakefulnessQ has also been reported in the late-gestation sheep fetus; it was characterized by REM sleep (which may contain other intermediate voltages and frequencies) coupled with the simultaneous appearance of vigorous somatic muscle activity, breathing movements, and the presence of eye activity [48,109,110,165,167,189,193,194,219]. The very brief and infrequent occurrence of this combination of behaviors typically only appears to occur as the fetus is transitioning into, or sometimes out of, NREM sleep, and this intermediate state appears to increase with gestational age [193,204,219,228] (Fig. 2). Curiously, in the fetal sheep literature at least, despite the simultaneous and almost constant occurrence of all of these behaviors earlier in gestation, at an age where brain maturation is equivalent to a 30-week-old human [148] and thus when nociceptive pathways may be intact (Fig. 1), fetuses are not referred to as being awake. 2.3.4. Evidence that the fetus remains asleep Certainly there is no consensus on whether this constellation of simultaneously occurring bawakeQ behaviors truly represents a distinct state and, if so, whether it represents wakefulness; that is, purposeful directed behavior as seen after birth [110,172,188]. Indeed, surprisingly little work has been done to explore the physiology of so-called fetal wakefulness, so that the existence of this state is accepted only because it is similar to newborn activity. However, an intriguing study by Rigatto and colleagues, in which the unanesthetized sheep fetus was observed directly in utero using a Plexiglas window, showed no evidence of fetal wakefulness, defined by open eyes and coordinated movements of the head, despite some 5000 h of recording [190]. Instead, fetal behavior alternated between the two basic behavioral states of REM and NREM sleep. Moreover, they considered that the polysynaptic reflexes they observed previously, which they had speculated might reflect wakefulness, were associated with generalized tonic discharge and rotation of the body and head during transition from REM to NREM sleep [189]. ARTICLE IN PRESS D.J. Mellor et al. / Brain Research Reviews xx (2005) xxx–xxx 5 Fig. 2. Example of a chart recorder tracing from a near-term sheep fetus at 124 days of gestation (0.8 gestation, term = 147 days). This stage of maturity corresponds with the human brain at term. The trace shows changes in fetal electroencephalogram (EEG), electrooculogram (EOG), nuchal electromyogram (EMG), diaphragm EMG, and tracheal pressure measurements. Note the clear presence of several episodes of a high voltage (low frequency) state, which is analogous to non-rapid eye movement (NREM) sleep, and a low voltage (high frequency) state, which is analogous to REM sleep. Fetal behavior at this age is now episodic in nature with fetal breathing movements and ocular activity typically occurring in REM sleep and body movements occurring in NREM sleep. The two arrows denote brief periods of a third state prior to a transition to NREM sleep, sometimes referred to as evidence of bwakefulnessQ. This state is characterized by eye, body, and breathing movements during either the end of a phase of REM sleep (first arrow) or during a short period of intermediate EEG amplitude (second arrow). We propose that this state corresponds with indeterminate or transitional sleep rather than a true state of wakefulness. The considered conclusion of this review, based on the analysis outlined below, and Rigatto’s important observations, is that the fetal behavior described by others as dwakefulnessT in fact represents a transition phase between sleep states, a state which in the newborn is called indeterminate sleep [65,157,202]. Thus, the increasing occurrence of this bstateQ with increasing fetal age is not because the fetus is more bawakeQ, but merely reflects the increased rate of switching between sleep states with advancing gestational age [50,170,220,228]. 2.4. Sleep transitions—an indeterminate state of sleep In both the term and preterm infant, active sleep segments are interrupted by periods of indeterminate or transitional sleep [202]. Indeed, preterm infants spend a considerable time in indeterminate sleep [133]. Transitional or indeterminate sleep is a state that cannot be definitely classified as active or quiet sleep and is comprised of mixed EEG activity, increased heart rate, blood pressure, and respiration, and behavioral changes including startlelike jerking and limb thrashing [82,92,157,202]. Importantly, this transitional sleep state does not represent wakefulness; it is not, for example, drowsiness [82]. We hypothesize that so-called wakefulness in utero is likely to represent transitional sleep in view of the behaviors described, its brief nature and temporal relationship to the onset or end of NREM sleep (Fig. 2), and its similarity to post-natal sleep-arousal. Full arousal from sleep (waking up) is a caudal–rostral brain process which originates in the ascending reticular activating system of the brainstem, spreads to the thalamus, and finally to the cortex. Importantly, however, arousal can also occur without cortical activation and waking; this is termed sleep-arousal. In the infant, sleep-arousal without waking is common [49,150,185]. The cardiorespiratory and postural changes during arousal do not represent cortical activation; rather, they are mediated by the brainstem [150,225]. In part, these periodically occurring reflexes serve cardiorespiratory homeostatic functions and also provide for several aspects of growth and development [225]. These functions are important, but critically, so too is sleep, particularly REM sleep, for normal neural maturation both pre- and postnatally [142,187]. Thus, during sleep-arousal, the caudal to rostral progression of impulses from the brainstem to the cortex is retarded by increasing inhibition which serves to decrease cortical arousal, thereby preserving the integrity of REM and NREM sleep, but at the same time allowing for essential cardiorespiratory and muscle activity [225]. ARTICLE IN PRESS 6 D.J. Mellor et al. / Brain Research Reviews xx (2005) xxx–xxx Arousing stimuli, such as obstructive apnea or tactile or auditory stimulation, can produce changes in cardiorespiratory activity and behavior (e.g., limb withdrawal), even in the absence of cortical EEG changes and waking [49,149]. These observations are consistent with the general concept that during sleep there is a blunted capacity to respond to external stimuli; i.e., stimuli may reach the cortex, but sensory information is bgatedQ. This is due to activitydependent depression of cortico-thalamic synapses produced by increased thalamic firing during arousal. Because cortico-thalamic cells are well coupled to inhibitory interneurons, this can result in enhanced cortical inhibition [41,83]. During indeterminate transitional sleep, corticothalamic responsiveness and thalamic transmission levels are the lowest of all sleep-waking stages [82]. Feedback inhibition in gating is mediated by a complex interaction of neurotransmitters [83], and the putative mechanisms of this enhanced inhibition in utero are discussed in depth below. In the newborn where sleep predominates, gating of sensory stimuli is likely to be a key mechanism helping to keep infants predominantly asleep [116,142]. As suggested by Roffwarg’s ontogenetic hypothesis, in the fetus REM sleep may in a large part substitute for wakefulness in the development of neural network organization, providing intense sensory input not otherwise available in utero [104,192]. Studies of fetal cerebral metabolism and activity during sleep states and altered oxygenation further support this contention [187]. Moreover, reduced sensory perception, as would be experienced in utero, acts to increase cortico-thalamic gating [130]. Postnatally, it is suggested that the decline in REM sleep after birth reflects the capacity to experience an awake state and the availability of sensory input to help the brain develop [104]. Collectively, these observations strongly suggest that the fetal behavior described as wakefulness is in fact that which is typically associated with changes in sleep states and is consistent with sleep-arousal. This does not equate with being awake. Further, it is not clear what purpose wakefulness would in fact serve for the fetus given the limited duration of this state and the limited sensory input available within the bdark and muffled confinesQ of the in utero environment [104]. However, it is clear that while sleep-arousal does not usually terminate in waking, full arousal can and does occur in the newborn, and this is an important part of the newborns defense against acute threats to its survival during sleep. Thus, noxious and nociceptive stimuli will awaken the newborn, but can the same stimuli awaken the fetus? 2.5. Can the fetus be woken up? If the fetus is essentially always asleep under physiological conditions, then can it be woken up by external nociceptive stimulation? This question has not been directly tested. In the newborn, a sufficiently threatening noxious stimulus (e.g., hypoxia or hypercapnia) will lead to full arousal, even in the preterm individual [135,150,173,225]. However, fetal responses to such stimuli are significantly different. The fetal response to hypoxia and severe asphyxia, for example, is characterized by apnea, cessation of fetal body movements, a shift to hypometabolic EEG states, and redistribution of combined ventricular output away from peripheral organs to key central organs such as the heart and brain [31,187,188,201]. Anatomically, some of these responses, such as the apneic response to hypoxia, are facilitated by unique inhibitory pathways within the fetal brain [53,79] and, as discussed below, cerebral metabolic changes are in a large part mediated by adenosine [107,120]. Similarly, while elevated CO2 tensions can elicit an increase in stimulated breathing movements if started in REM sleep, this response is inhibited when the fetus transitions into NREM sleep [191], and this is in part due to the inhibitory effect of temperature in utero [126]. These data demonstrate that stimuli which induce arousal to waking postnatally do the opposite in utero; they further suppress fetal arousal, and for that reason, promote survival of the fetus during adverse conditions. This inhibitory set of responses is a key defense strategy to conserve energy [31,187]. These responses to hypoxia also exist in very young fetuses [31,70,115], although the greater anaerobic reserves of the younger fetus appear to permit a brief period of intense movements at the onset of a severe asphyxial insult which may help the fetus extricate itself from harm [70]. However, these movements occur despite the cortical EEG switching to an isoelectric state, i.e., a state of profound suppression [30]. Thus, they are likely to be subcortically/brainstem mediated and do not reflect arousal to an awake state. This is despite the life-threatening severity of the insult and the greater quantities of cerebral energy reserves in the preterm fetus which could potentially be utilized to transition to an awake state [51,206], if such a state was important for survival. These observations are important because they demonstrate that the in utero environment is not the same as that in which the newborn lives, and this necessitates different responses from the fetus, mediated in part by unique wiring of the fetal brain. Unlike the situation postnatally, arousal costs the fetus critically limited energy resources and for little advantage. Further evidence that fetal arousal in response to noxious stimuli is suppressed is suggested by studies of the intense and potentially bpainful or distressingQ stimuli used in vibroacoustic stimulation (VAS). The purpose of VAS is to evaluate the integrity of the fetal central nervous system by eliciting distinct fetal movements or fetal heart rate changes [1]. It is accompanied by variable changes in EEG activity, depending on the fetal dsleep stateT during stimulation [2,23, 131,234]. Recent detailed analysis of the EEG, however, shows similar dynamics to those seen during spontaneous sleep state transitions rather than arousal to an awake state [204]. These data suggest the effects of VAS are mediated by actions of the brainstem structures and activation of the afferent reticular arousal system [204]. These findings are consistent with the observations that in the newborn cortical ARTICLE IN PRESS D.J. Mellor et al. / Brain Research Reviews xx (2005) xxx–xxx arousal can occur in response to stimuli, without wakefulness occurring [149,225]. 7 typically characterized by greater inhibition. If the fetus is always asleep, it is not conscious, and therefore cannot experience nociceptive inputs as pain. 2.6. Can the fetus dream? We note in passing that these observations bear on the question of whether the fetus can be said to bdreamQ. Suffice to say the subject of how and why we dream remains a complex and hotly debated field of research: for example, whether it has a role in consolidating conscious experiences such as learning and memory, or do we dream in order to forget [216,231]? To ask whether the fetus dreams is an equally difficult issue to address. The inherent psychoanalytical issues aside, dreams (at least postnatally) are composed in a large part by memories obtained in the awake state. Sleep itself, however, is often referred to as an bamnesiac stateQ: the content of sleep is poorly remembered, suggesting that the structures responsible for encoding and storing information are suppressed or absent in sleep [231]. This in turn suggests that new memories, at least declarative memories (the type of memory commonly referred to by the terms bmemoryQ or brememberingQ [231]), are not likely to be obtained during sleep. From this perspective, due caution should be given to the hypothesis that fetuses can learn in utero and that memories based on this learning can be recalled after birth. It may be argued that neural entrainment to a repeated stimulus (such as noise) which produces a reflex response to that stimulus, as has been demonstrated recently in chimpanzee newborns after repeated exposure in utero to sound [114], is not the same as cognitive awareness or learning about a stimulus to produce declarative memory. Whether procedural or implicit memory (memory formed by unconscious acquisition and utilization of perceptual and motor skills) truly occurs in utero, or more importantly is a source for dreams, can only be speculated upon. On the whole, however, given the apparent importance for dreaming of memory obtained in the awake state, the general amnesiac aspect of sleep and that the fetus appears to be actively maintained asleep, fetal dreaming seems unlikely. 2.7. Summary of bfetal wakefulnessQ In summary, this evaluation of the clinical and experimental literature shows that there is no convincing evidence to suggest that the fetus is ever awake; rather, it supports the concept that the fetus exists in a continuous sleep-like state. The evidence suggests, further, that so-called fetal wakefulness, which is said to occur between REM and NREM sleep states, is more akin to transitional or indeterminate sleep or to non-waking cortical arousal rather than an awake state. Although a state of cortical arousal can be elicited by external stimuli, it is a continuation of sleep. Indeed, evidence suggests that unlike the newborn, noxious or nociceptive stimuli do not cause cortical arousal to an awake state as a defense response; rather, the response of the fetus is 3. Suppressors of fetal behavior and cortical activity The conclusion suggested in the section above is further strengthened by consideration of the increasing body of evidence which shows that there are several suppressors in utero which act to inhibit neural activity in the fetus to a far greater degree than is seen postnatally in the infant. The uterus plays a key role in providing the chemical and physical factors that together help to keep the fetus continuously asleep. We propose that this is achieved, among other things, through the combined neuroinhibitory actions of a powerful EEG suppressor and sleep inducing agent (adenosine), two neurosteroidal anesthetics (allopregnanolone, pregnanolone) and a potent sleep-inducing hormone (prostaglandin D2), acting together with a putative peptide inhibitor and other factors produced by the placenta, further supported by the warmth and cushioned tactile stimulation of the uterine environment. When considering this concept, it is important to distinguish between overall neural activity and the maturation of local gating at the spinal level. For example, there is evidence from the neonatal rat that descending inhibition is markedly less than in the adult. This immaturity is suggested to reflect either delayed maturation of crucial interneurons in the dorsal horn or insufficient local levels of 5-hydroxytryptamine [67], and not overall neural activity. A proposed schema of the putative inhibitory factors can be seen in Fig. 3. In this section, we review the fetal EEG and fetal behavioral evidence for a key role for these neurosuppressors. 3.1. Adenosine and oxygen status Adenosine, a purinergic messenger, regulates many physiological processes in excitable tissues, especially the brain, by inhibiting metabolic activity and/or by modulating the supply of metabolic substrates via vasodilatation [61,169,180]. Adenosine, acting via its A1 receptors, inhibits many neurotransmitters, especially the excitatory glutaminergic systems, so that the net effect in nearly every region of the brain is to reduce excitability [61]. This is relevant to sleep and arousal states, as high circulating and tissue concentrations of adenosine promote sleep [169,180]. The biological half-life of adenosine is very short, well under 10 s [34,214], typically in the range of 0.6–1.5 s [159], so that the plasma and tissue concentrations of adenosine change very rapidly in response to changes in adenosine production. In the sheep and human being, the circulating concentrations of adenosine are 2- to 4-fold higher in the fetus than the dam [20,153,200,239–241]. Although all tissues may contribute to circulating adenosine, the placenta and to a ARTICLE IN PRESS 8 D.J. Mellor et al. / Brain Research Reviews xx (2005) xxx–xxx gen status and adenosine concentrations [20,21,153,196, 200,238,239]. Similarly, adenosine concentrations rise during hypoxia–ischaemia in the perinatal brain before the onset of anoxic depolarization [176]. These findings indicate that the relatively high levels of adenosine in the fetus, compared with those postnatally, are derived primarily from the placenta. They indicate further that variations due to changes in fetal oxygen tensions are superimposed on these high levels, such that fetal hypoxemia further elevates and hyperoxemia reduces adenosine levels. Finally, they show that the high adenosine levels, augmented at times by hypoxemia, contribute to maintaining the fetus in sleep. 3.2. Neuroactive steroids—allopregnanolone and pregnanolone Fig. 3. Flow diagram illustrating the production of inhibitory neurotransmitters both within the brain and in the placenta that help to suppress fetal awareness. The physical characteristics of the fetal environment such as its relative warmth and low absolute oxygen tensions also contribute to this state, at least in part by augmenting fetal adenosine levels, and potentially other inhibitory factors as well. lesser extent probably the fetal liver are the major sources [20,21,138,200,209]. Fetal plasma adenosine concentrations increase further during hypoxemia or anemia in human fetuses [196,238,239,240] and in fetal sheep [120,123] and decrease during marked hyperoxemia in fetal sheep [20,21, 153,200]. The increase in local adenosine during severe hypoxia suppresses cerebral metabolism and helps limit neural injury [107]. As in the adult, fetal adenosine levels affect sleep state and arousal as reflected in REM-related and NREM-related EEG activity, muscular activity, and breathing movements. Infusion of adenosine reduces the incidence of rapid eye movements and fetal breathing during the first hour [120– 122], but these effects are not sustained during 9-h infusions [121]. The metabolically stable adenosine analogue, 1phenylisopropyl-adenosine, reduces the incidence of REMrelated and increases the incidence of NREM-related EEG activity in a dose-dependent manner, and abolishes fetal breathing and muscular activity [221]. Conversely, infusions of theophylline, a non-specific adenosine receptor antagonist, increase fetal breathing and the incidence of REM sleep [18], and abolish the suppressive effects of adenosine infusion [121]. These data demonstrate that the higher concentrations of adenosine in fetal than in maternal plasma contribute to the predominance of sleep-like EEG activity seen before birth in the human and sheep fetus [32,44,52,93,226]. Moreover, the effects of short-term and long-term hypoxemia [37,38] and of hyperoxemia [19,95] on EEG activity, rapid eye movement, breathing, and body movements in fetal sheep seem to primarily reflect the inverse relationship between fetal oxy- Pregnane metabolites of progesterone which contain a 3a-hydroxyl group include allopregnanolone (5a-pregnane3a-ol-20-one) and pregnanolone (5h-pregnane-3a-ol-20one), and have potent sedative/hypnotic and anaesthetic effects [139,156,175]. They enhance g-amino-butyric-acid (GABAA) receptor binding [47], thereby increasing the activity of GABA inhibitory pathways in the central nervous system (CNS) [139]. These pregnane metabolites are primarily synthesized within the CNS from cholesterol, via progesterone, by 5a-reductase-mediated conversion [178], but may also be synthesized from progesterone produced elsewhere, and circulating pregnanes synthesized in other tissues readily cross the blood–brain barrier [24,47,134]. Given their demonstrated CNS-suppressive effects, 3ahydroxyl pregnanes are strongly implicated as inhibitory modulators of fetal EEG activity and behavioral state. Similar to adenosine, the placenta produces large quantities of progesterone [22] and possibly pregnanes throughout at least the last half of pregnancy [163]. Thus, plasma concentrations and brain concentrations of progesterone and the pregnanes fall markedly after birth [59,111,163,178,205]. GABAA receptors are present in the brain of fetal sheep from at least 56 days of gestation [232]. During the last 20– 30 days of pregnancy, injections of progesterone or its 3ahydroxyl metabolites into the fetal circulation or cerebral ventricles reduce fetal EEG activity and eye, breathing, and body movements, and conversely, inhibition of placental production of progesterone or its metabolites enhances these activities [48,102,164–167]. Similarly, inhibition of GABAA by picrotoxin increases fetal EEG, breathing, and behavioral activity [113,166]. Consistent with data from the sheep fetus, 3a-hydroxyl pregnanes, produced from progesterone in fetal CNS and other tissues, can act on GABAA receptors in the human fetal brain [134]. The placenta produces large quantities of progesterone throughout the last half of human pregnancy (and likely earlier) [60,99]. Progesterone and its 3a-hydroxyl metabolites have been detected in human fetal plasma [36,101,119,155] and 5a-reductase is present ARTICLE IN PRESS D.J. Mellor et al. / Brain Research Reviews xx (2005) xxx–xxx from at least 17 weeks of gestation in the human fetal CNS, skin, liver, kidneys, lungs, and small intestines [197]. Moreover, allopregnanolone and pregnanolone are present at significant concentrations in umbilical blood at birth in the human infant [36,101]. In stress situations such as hypoxia, allopregnanolone levels are actively maintained in the brain and are increased in circulating plasma [163]. 3.3. Prostaglandin D2 Prostaglandin D2 (PGD2) is a potent sleep-inducing paracrine hormone which is formed in the adult mammalian CNS from prostaglandin H2 under the action of the enzyme prostaglandin D synthase (PGDS) [96]. Sleep induced by intracerebroventricular (icv) infusion of PGD2 is indistinguishable from natural sleep as judged by its normal REM and NREM characteristics and by several other electrophysiological and behavioral criteria [96]. PGD2 infused into the subarachnoid space promotes sleep by increasing the firing rate of sleep-active neurons in the ventrolateral preoptic area, adjacent to the anterior hypothalamus, and not elsewhere in the brain [96]. The mechanisms of action of PGD2 involve paracrine stimulation of adenosine release which leads to GABAergic inhibition of wake-promoting neurons in the posterior hypothalamus [97]. PGDS is present in the cerebrospinal fluid of fetal sheep at 125 and 135 days of gestation, but not at 90 days [132]. Inhibition of PGDS action by icv infusion of SeCl4 increases fetal behavioral activity as indicated by increased nuchal muscle, electroocular, and breathing activity, and icv infusion of PGD2 reverses these SeCl4-induced effects [132]. These observations suggest that PGD2 has a basal role in inducing sleep in fetal sheep from at least 125 days of gestational, when discrete REM and NREM sleep states are established [52,193,194]. 3.4. Placental peptide inhibitor and other factors A key role for the placenta in maintaining fetal sleep states is shown by studies of bbirth in uteroQ, in which the fetus is ventilated to achieve normal oxygenation. Under these conditions, when the umbilical cord is occluded, continuous behavioral activity and continuous breathing develop in the fetal lamb [9–11]. This paradigm mimics the effects of separation from the placenta at birth, and thus removal of its inhibitory effects. Placental adenosine and pregnanes are likely important mediators of this effect, but there is some evidence for an additional placental inhibitory factor. Infusion of a placental extract but not extracts of other fetal tissues suppresses fetal activity and respiration within 2 min [9– 11]. This putative factor has not been fully characterized, but is probably a peptide with a molecular mass between 2.5 and 4.5 kDa. However, not all studies support the existence of such a placental mediator [125]. 9 Other inhibitors are also likely to contribute, for example, neuropeptide Y (NPY), somatostatin, enkephalins, and corticotropin releasing hormone (CRH) [100,213]. Postnatally, NPY is known to have sleep promoting and sedative actions and to reduce pain [161,235]. The levels of NPY are relatively high in the fetal brain and decline after birth [100], and during fetal life are known to play a role in fetal responses to asphyxia [199]. Other peptides, such as substance P, which is a primary sensory transmitter mediating pain sensations via the thin C-fibers, markedly increase expression and function after birth [33,184]. While CRH is well known to be produced during stress and mediates many of its effects, it is less well appreciated that it also has analgesic effects at all levels of the neuraxis [129], which may augment the overall protective role of this system during stress. Likewise, placental CRH in human beings is speculated to play a role in mediating the rapid attenuation of fetal responses to repeated stimuli [198], and needling procedures appear to stimulate CRH release from placental not fetal sources [75]. In the late-gestation sheep fetus CRH is known to play a critical role in modulating the episodic nature of fetal behavior [29]. Also, endogenous activity in the kappa opioid system may be functional in modulating responses to the sensory environment; in particular, it may regulate the rapid fetal habituation to sensory input [213]. Finally, growth hormone (GH) levels are high in fetal life, but unlike the situation postnatally, GH does not significantly contribute to fetal growth [108]. There is a positive relationship, however, between GH and sleep [227], and sleep-related GH secretion is probably caused by an increased production of hypothalamic growth hormonereleasing hormone acting on the pituitary [227]. Speculatively, the high levels of GH seen during fetal life may reflect an important role for growth hormone in keeping the fetus asleep. A similar role may be postulated for ghrelin, an endogenous ligand for the growth hormone secretagogue receptor, which may be secreted both by the fetus and the placenta [117,229]. 3.5. Thermal status—warmth Sleep-wakefulness and body temperature are known to influence each other. Postnatally, we know that warmth can make us drowsy; sleep is effectively promoted by a hot bath, for example [105]. There is evidence that fetal thermal status affects the level of behavioral activity in utero, i.e., that warmth suppresses it. The work by Kuipers and colleagues suggests that temperature modulates the state-related fetal respiratory response to elevated CO2 tensions [126]. Human, sheep, and other fetuses are slightly hyperthermic in relation to their mothers because the heat they generate can only be dissipated down a thermal gradient across the placenta [3,80,181]. Cooling mature well-oxygenated fetal sheep in utero, such that their cutaneous thermoreceptors are exposed to cold via cooling coils in amniotic fluid, elicits behavioral activity, shivering, and increased respiration, and ARTICLE IN PRESS 10 D.J. Mellor et al. / Brain Research Reviews xx (2005) xxx–xxx rewarming such fetuses reverses these effects [80,88– 90,181]. In utero cooling of the fetal skin using amniotic fluid cooling coils apparently induces EEG changes indicative of arousal [203]. Immersing aroused, physically active, and conscious newborn lambs in water at maternal body temperature induces a sleep-like, non-aroused, unconscious state, and cooling the water restores their prior aroused, conscious, and physically active state [62]. The translation of thermal information from the skin to induction of sleep may occur via temperature-sensitive neurons within the hypothalamus [147,154]. Various regions of the hypothalamus (e.g., the hypothalamic preoptic area and prefrontal areas of the lateral hypothalamus) are involved in both the regulation of sleep-wakefulness and body temperature [147,154]. Activation of sleep-related warm sensitive neurons and the deactivation of wake-related cold-sensitive neurons appear to play a role in the onset and regulation of NREM sleep [6]. Further, warmth inhibits neuronal discharge in arousal-related brain structures, including regions which promote wakefulness such as the dorsal raphe nucleus [6,91,147]. These responses may be mediated by multiple inhibitors such as adenosine, GABA, prostaglandins, and growth hormone [7,98,145,218,244]. In this way, the constant moderate ambient warmth experienced by the fetus may facilitate a constant state of sleep. 3.6. Tactile stimulation Tactile sensory input is minimized in utero by amniotic fluid which buffers the embryo/fetus against mechanical stimulation and presumably also reduces sensations associated with gravity. In contrast, intense tactile stimulation during labor and after delivery is considered to contribute to the usually rapid onset of behavioral activity and consciousness in newborn human infants [127,128] and other newborn animals, including lambs [151]. 4. Long-term sequelae Here we consider one final issue: whether nociceptive inputs may have deleterious consequences even if the bendogenously anesthetizedQ fetus does not consciously perceive pain at the time of stimulation. Can exposure to noxious stimuli initiate a cascade of events that sensitize the nervous system [84], or can repeated pain exposure in preterm infants contribute to attention, learning, and behavior problems later in life [35,87,237]? It is critical to appreciate that not only is most of the available information based on studies in preterm infants, and not the fetus, but also there are no direct studies which have robustly tested these speculations, even in the preterm newborn, and thus no empirical data to demonstrate a causal relationship. It is striking that where bstressQ or treatment with steroids has been found to affect the fetus [45,46,55,86,162], the bstressQ has been chronic, often extending over a third or more of gestation, whereas brief treatment seems to affect development only when given very early in gestation [58]. Moreover, the fetus is likely to be naturally exposed to elevations in bstressQ hormones, for example, during periods of hypoxia or other stresses [77]. Such exposure may occur frequently throughout development, particularly during early to mid-gestation [27,70], and circulating cortisol concentrations increase markedly in late-gestation, staying elevated until several weeks after birth [144]. Thus, it is likely that a brief period of fetal hypothalamic–pituitary– adrenal (HPA) activation in mid- to late gestation in response to short duration surgery-induced nociceptor input would have limited importance. In summary, considerably more work is required to evaluate the long-term consequences of nociceptor activation in the fetus and indeed the premature newborn. Until then, we, like Grunau, advise against overinterpreting correlational studies and limited data [87]. 3.7. Summary of endogenous inhibition 5. Summary and conclusions This analysis suggests that the suppression of fetal wakefulness-related CNS activity and behavior described in the first part of this review is achieved to a significant extent by the combined effects of multiple neurotransmitters, peptides, and endocrine factors coupled with warmth, buoyancy, and cushioned tactile stimulation (Fig. 3). During stress situations, CNS suppressor effects are often significantly increased, and this leads to an even deeper inhibition of the fetus, providing, we argue, a greater degree of bendogenous anesthesiaQ. Clearly much more work is required to dissect the relative importance of each of these systems and to understand fully how the systems interact. Nevertheless, the existing data strongly suggest that the fetal response to noxious and nociceptive stimuli is significantly different from that seen after birth and is in large part due to its unique environment, with the placenta playing a key role. We have considered whether the fetus, once its nociceptive pathways are complete, can feel pain in utero in a psychological manner akin to adult pain experience, and whether regardless of this the physiological responses to nociceptive input may lead to altered behavior later in life. We conclude that there is currently no strong evidence to suggest that the fetus is ever awake, even transiently; rather, it is actively kept asleep (and unconscious) by a variety of endogenous inhibitory factors. Thus, despite the presence of intact nociceptive pathways from around mid-gestation, the critical aspect of cortical awareness in the process of pain perception is missing. Furthermore, there is currently no direct evidence to suggest that subcortical effects of nociceptor input in the fetus can alone alter neural development and cause long-term adverse outcomes. Rather, the inference that this may occur is derived from studies of ARTICLE IN PRESS D.J. Mellor et al. / Brain Research Reviews xx (2005) xxx–xxx chronic maternal bstressQ or administration of synthetic glucocorticoids, or speculation about the potential vulnerability of immature neurons. It is of concern that such hypotheses about the impact of nociceptive stimuli are readily accepted as fact and that this perception in turn instructs our clinical understanding about the management of presumed pain in utero. The influence of the postnatal environment and of cognition and learning on modulating these effects is clearly quite considerable; an influence that has not been considered in most studies and reviews. Furthermore, key questions have typically not been addressed when considering the potential impact of presumed pain in utero. For example, what is the interrelationship between the relatively diffuse immature pain pathways [136], and the overall inhibition from endogenously-produced analgesic neurotransmitters during noxious stimuli? Another example is that the stress response to noxious stimuli includes release of CRH which has analgesic effects in its own right in the fetus but not postnatally [39,160]. The fetal response to nociceptive input is clearly more complex than we currently appreciate, and as highlighted above appears to be dengineeredT to further anesthetize the fetus during exposure to noxious stimuli. It may be strongly argued that these factors interact to significantly ameliorate, if not eliminate, the deleterious impact of nociceptive input in the fetus compared to the effects postnatally. Surprisingly, despite advocacy for analgesic use during fetal surgery, the impact of analgesics and anesthetics on brain and other organ development has not been extensively studied. Yet there is good evidence to show that such agents affect the fetus quite differently and may cause fetal compromise. In fetal sheep, morphine, for example, is not a cardiorespiratory depressant; rather, it induces continuous fetal breathing movements (FBMs) which increase metabolic demand [28,42]; it also stimulates the fetal HPA axis [223] and alters fetal glucose handling [222]. In utero, opiates do not necessarily have analgesic properties and may increase fetal compromise during stress situations [124,141,212]. Importantly, there is now evidence to show that exposure of the fetus to opiates may cause significant neuronal and white matter cell loss [81,106,207,217]. Non-narcotic analgesic agents have also not been evaluated extensively [177], but may have similar consequences. For example, indomethacin also significantly increases FBMs [4] and may alter the fetal responses to hypoxia [103]. In newborn immature rats, at least indomethacin administration is associated with subsequent impaired neural development [26]. For all agents, given the diffuse nature of pain receptivity and the limited fetal capacity to metabolize many agents, it is difficult to judge dosage and the required duration of treatment, and it is difficult to find a safe route which can be used repeatedly or chronically. In the adult, some experimental studies have indicated that pre-emptive analgesia which blocks the intense afferent barrage of impulses received by the subcortex and brainstem 11 during surgery significantly modifies post-surgical pain [152]. These findings are, however, not supported by a recent major meta-analysis of clinical trials, mainly in adults, which found no evidence that pre-operative analgesia can substantially reduce post-injury pain hypersensitivity [158]. Although there are few large studies in preterm newborns, a randomized controlled trial of pre-emptive analgesia with morphine was associated with worse outcomes [14], no appreciable change in pain scores [208], and no reduction in adverse neurological outcome [14,208]. Indeed, there is some evidence that prolonged exposure to analgesic drugs may detrimentally alter neuronal and synaptic organization permanently [12]. What we hope is apparent from this review is that this important issue is clouded by inappropriate extrapolation from the postnatal state to the fetal condition. The assumption that the newborn can act as a surrogate for the fetus is wrong, for it denies that the environment in which the fetus lives is unique, and that the fetus itself responds quite differently to many challenges. Greater scientific rigor is required to directly address the concept of fetal awareness in utero as part of understanding presumed fetal pain perception, and of the potential impact of nociceptor input on neural development and subsequent behavior. It is not enough to say, bIf in doubt treatQ, for treatment may produce greater adverse outcomes than the effects of relatively transient nociceptor input. Certainly, it is the conclusion of this review that in order to better understand the nature of presumed fetal pain, we must first evaluate the fetus and its unique in utero environment from the viewpoint of the fetus; not as a newborn not yet born or as an adult who is simply waiting to grow up. Acknowledgments The authors gratefully acknowledge several organizations which provided funding that directly or indirectly supported the completion of this review: the Auckland Medical Research Council, the Health Research Council of New Zealand, the National Institutes of Health (RO1 HD32752), the Institute of Food, Nutrition and Human Health and the Riddet Centre (Massey University), and the Ministry of Agriculture and Forestry. We also wish to thank Drs. Craig Johnson (Massey University, New Zealand) and David Walker (Monash University, Australia) for helpful advice and comments. References [1] R.M. Abrams, K.J. Gerhardt, The acoustic environment and physiological responses of the fetus, J. Perinatol. 20 (2000) S31 – S36. [2] R.M. Abrams, M. Schwab, K.J. Gerhardt, R. Bauer, A.J. Peters, Vibroacoustic stimulation with a complex signal: effect on behavioral state in fetal sheep, Biol. Neonate 70 (1996) 155 – 164. ARTICLE IN PRESS 12 D.J. Mellor et al. / Brain Research Reviews xx (2005) xxx–xxx [3] K.J. Adamsons, M.E. Towell, Thermal homeostasis in the fetus and newborn, Anesthesiology 26 (1965) 531 – 548. [4] S.L. Adamson, D. Engelberts, K.J. Whiteley, Low dose indomethacin via fetal vein or cerebral ventricle stimulates breathing movements in fetal sheep, Can. J. Physiol. Pharmacol. 75 (1997) 135 – 142. [5] T. Akerstedt, M. Billiard, M. Bonnet, G. Ficca, L. Garma, M. Mariotti, P. Salzarulo, H. Schulz, Awakening from sleep, Sleep Med. Rev. 6 (2002) 267 – 286. [6] M.N. Alam, D. McGinty, R. Szymusiak, Thermosensitive neurons of the diagonal band in rats: relation to wakefulness and non-rapid eye movement sleep, Brain Res. 752 (1997) 81 – 89. [7] M.N. Alam, R. Szymusiak, H. Gong, J. King, D. McGinty, Adenosinergic modulation of rat basal forebrain neurons during sleep and waking: neuronal recording with microdialysis, J. Physiol. 521 (Pt. 3) (1999) 679 – 690. [8] T.F. Almeida, S. Roizenblatt, S. Tufik, Afferent pain pathways: a neuroanatomical review, Brain Res. 1000 (2004) 40 – 56. [9] R. Alvaro, V. de Almeida, S. Al-Alaiyan, M. Robertson, B. Nowaczyk, D. Cates, H. Rigatto, A placental extract inhibits breathing induced by umbilical cord occlusion in fetal sheep, J. Dev. Physiol. 19 (1993) 23 – 28. [10] R.E. Alvaro, V.d.A. Rehan, V.Z. Haider, M. Robertson, A. Jansen, D.B. Cates, H. Rigatto, Specificity of a placental factor inhibiting breathing in fetal sheep, Reprod. Fertil. Dev. 8 (1996) 423 – 429. [11] R.E. Alvaro, M. Robertson, S. Al-Saedi, R.P. Lemke, D.B. Cates, H. Rigatto, Preliminary characterization of a placental factor inhibiting breathing in fetal sheep, Reprod. Fertil. Dev. 9 (1997) 641 – 649. [12] K.J. Anand, Effects of perinatal pain and stress, Prog. Brain Res. 122 (2000) 117 – 129. [13] K.J.S. Anand, D.B. Carr, The neuroanatomy, neurophysiology, and neurochemistry of pain, stress, and analgesia in newborns and children, Pediatr. Clin. North Am. 36 (1989) 795 – 822. [14] K.J. Anand, R.W. Hall, N. Desai, B. Shephard, L.L. Bergqvist, T.E. Young, E.M. Boyle, R. Carbajal, V.K. Bhutani, M.B. Moore, S.S. Kronsberg, B.A. Barton, Effects of morphine analgesia in ventilated preterm neonates: primary outcomes from the NEOPAIN randomised trial, Lancet 363 (2004) 1673 – 1682. [15] K. Andrews, M. Fitzgerald, The cutaneous withdrawal reflex in human neonates: sensitisation, receptive fields, and the effects of contralateral stimulation, Pain 56 (1994) 95 – 101. [16] Anonymous, Report of the MRC expert group on fetal pain, In: M.R. Council (Ed.), Medical Research Council, London, 2001. [17] D. Arduini, G. Rizzo, C. Giorlandino, H. Valensise, S. Dell’Acqua, C. Romanini, The development of fetal behavioural states: a longitudinal study, Prenat. Diagn. 6 (1986) 117 – 124. [18] A. Avital, A.H. Jansen, D.S. Sitar, V. Chernick, Influence of prolonged adenosine receptor blockade on fetal sleep and breathing patterns, Respir. Physiol. 91 (1993) 227 – 236. [19] R.J. Baier, S.U. Hasan, D.B. Cates, B. Hooper, B. Nowaczyk, H. Rigatto, Effects of various concentrations of O2 and umbilical cord occlusion on fetal breathing and behavior, J. Appl. Physiol. 68 (1990) 1597 – 1604. [20] K.T. Ball, T.R. Gunn, G.G. Power, H. Asakura, P.D. Gluckman, A potential role for adenosine in the inhibition of non-shivering thermogenesis in the fetal sheep, Pediatr. Res. 37 (1995) 303 – 309. [21] K.T. Ball, T.R. Gunn, P.D. Gluckman, G.G. Power, Suppressive action of endogenous adenosine on ovine fetal nonshivering thermogenesis, J. Appl. Physiol. 81 (1996) 2393 – 2398. [22] J.M. Bassett, T.J. Oxborrow, I.D. Smith, G.D. Thorburn, The concentration of progesterone in the peripheral plasma of the pregnant ewe, J. Endocrinol. 45 (1969) 449 – 457. [23] R. Bauer, M. Schwab, R.M. Abrams, J. Stein, K.J. Gerhardt, Electrocortical and heart rate response during vibroacoustic stimulation in fetal sheep, Am. J. Obstet. Gynecol. 177 (1997) 66 – 71. [24] E.E. Baulieu, Neurosteroids: of the nervous system, by the nervous system, for the nervous system, Recent Progr. Horm. Res. 52 (1997) 1 – 32. [25] D. Benatar, M. Benatar, A pain in the fetus: toward ending confusion about fetal pain, Bioethics 15 (2001) 57 – 76. [26] O. Benesova, H. Tejkalova, Z. Kristofikova, P. Husek, J. Nedvidkova, A. Yamamotova, Brain maldevelopment and neurobehavioural deviations in adult rats treated neonatally with indomethacin, Eur. Neuropsychopharmacol. 11 (2001) 367 – 373. [27] L. Bennet, A.J. Gunn, Fetal origins of adult mental illness, in: E.M. Wintour-Coghlan, J.A. Owens (Eds.), Early life origins of adult health and disease, Landes Bioscience, Georgetown, TX, 2004. [28] L. Bennet, B.M. Johnston, P.D. Gluckman, The central effects of morphine on fetal breathing movements in the fetal sheep, J. Dev. Physiol. 8 (1986) 297 – 305. [29] L. Bennet, B.M. Johnston, W.W. Vale, P.D. Gluckman, The effects of corticotrophin-releasing factor and two antagonists on breathing movements in fetal sheep, J. Physiol. 421 (1990) 1 – 11. [30] L. Bennet, S. Rossenrode, M.I. Gunning, P.D. Gluckman, A.J. Gunn, The cardiovascular and cerebrovascular responses of the immature fetal sheep to acute umbilical cord occlusion, J. Physiol. 517 (1999) 247 – 257. [31] L. Bennet, J.A. Westgate, P.D. Gluckman, A.J. Gunn, Fetal responses to asphyxia, in: D.K. Stevenson, P. Sunshine (Eds.), Neonatal brain injury: mechanisms, management, and the risks of practice, Cambridge Univ. Press, Cambridge, 2003, pp. 83 – 114. [32] P.J. Berger, A.M. Walker, R. Horne, V. Brodecky, M.H. Wilkinson, F. Wilson, J.E. Maloney, Phasic respiratory activity in the fetal lamb during late gestation and labour, Respir. Physiol. 65 (1986) 55 – 68. [33] L. Bergstrom, H. Lagercrantz, L. Terenius, Post-mortem analyses of neuropeptides in brains from sudden infant death victims, Brain Res. 323 (1984) 279 – 285. [34] R.M. Berne, Adenosine: an important physiological regulator, News Physiol. Sci. 1 (1986) 163 – 167. [35] A.T. Bhutta, K.J. Anand, Vulnerability of the developing brain. Neuronal mechanisms, Clin. Perinatol. 29 (2002) 357 – 372. [36] M. Bicikova, J. Klak, M. Hill, Z. Zizka, R. Hampl, P. Calda, Two neuroactive steroids in midpregnancy as measured in maternal and fetal sera and in amniotic fluid, Steroids 67 (2002) 399 – 402. [37] A.D. Bocking, R. Harding, Effects of reduced uterine blood flow on electrocortical activity, breathing, and skeletal muscle activity in fetal sheep, Am. J. Obstet. Gynecol. 154 (1986) 655 – 662. [38] A.D. Bocking, R. Gagnon, K.M. Milne, S.E. White, Behavioural activity during prolonged hypoxemia in fetal sheep, J. Appl. Physiol. 65 (1988) 2420 – 2426. [39] A.I. Bogdanov, N.I. Yarushkina, Mechanisms of the effects of adrenocorticotropic hormone on pain sensitivity in rats, Neurosci. Behav. Physiol. 33 (2003) 795 – 798. [40] J.A. Burgess, S.A. Tawia, When did you first begin to feel it?— locating the beginning of human consciousness, Bioethics 10 (1996) 1 – 26. [41] M.A. Castro-Alamancos, Absence of rapid sensory adaptation in neocortex during information processing states, Neuron 41 (2004) 455 – 464. [42] P.Y. Cheng, D. Wu, Y. Soong, S. McCabe, J.A. Decena, H.H. Szeto, Role of mu 1- and delta-opioid receptors in modulation of fetal EEG and respiratory activity, Am. J. Physiol. 265 (1993) R433 – R438. [43] F.A. Chervenak, L.B. McCullough, D.J. Birnbach, Ethical issues in fetal surgery research, Best Pract. Res., Clin. Anaesthesiol. 18 (2004) 221 – 230. [44] F. Clewlow, G.S. Dawes, B.M. Johnston, D.W. Walker, Changes in breathing, electrocortical and muscle activity in the unanaesthetized fetal lamb with age, J. Physiol. 341 (1983) 463 – 476. [45] C.L. Coe, G.R. Lulbach, M.L. Schneider, Prenatal disturbance alters the size of the corpus callosum in young monkeys, Dev. Psychobiol. 41 (2002) 178 – 185. [46] C.L. Coe, M. Kramer, B. Czeh, E. Gould, A.J. Reeves, C. Kirschbaum, E. Fuchs, Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile rhesus monkeys, Biol. Psychiatry 54 (2003) 1025 – 1034. ARTICLE IN PRESS D.J. Mellor et al. / Brain Research Reviews xx (2005) xxx–xxx [47] N.A. Compagnone, S.H. Mellon, Neurosteroids: biosynthesis and function of these novel neuromodulators, Front. Neuroendocrinol. 21 (2000) 1 – 56. [48] K.J. Crossley, M.B. Nicol, J.J. Hurst, D.W. Walker, G.D. Thorburn, Suppression of arousal by progesterone in fetal sheep, Reprod. Fertil. Dev. 9 (1997) 767 – 773. [49] R.J. Davies, P.J. Belt, S.J. Roberts, N.J. Ali, J.R. Stradling, Arterial blood pressure responses to graded transient arousal from sleep in normal humans, J. Appl. Physiol. 74 (1993) 1123 – 1130. [50] G.S. Dawes, The 1987 James A.F. Stevenson Memorial Lecture. The development of fetal behavioural patterns, Can. J. Physiol. Pharmacol. 66 (1988) 541 – 548. [51] G.S. Dawes, J.C. Mott, H.J. Shelley, The importance of cardiac glycogen for the maintenance of life in foetal lambs and newborn animals during anoxia, J. Physiol. 146 (1959) 516 – 538. [52] G.S. Dawes, H.E. Fox, B.M. Leduc, G.C. Liggins, R.T. Richards, Respiratory movements and rapid eye movement sleep in the foetal sheep, J. Physiol. 220 (1972) 119 – 143. [53] G.S. Dawes, W.N. Gardner, B.M. Johnston, D.W. Walker, Breathing in fetal lambs: the effect of brain stem section, J. Physiol. 335 (1983) 535 – 553. [54] J.I. de Vries, G.H. Visser, H.F. Prechtl, The emergence of fetal behaviour: III. Individual differences and consistencies, Early Hum. Dev. 16 (1988) 85 – 103. [55] C. de Weerth, Y. van Hees, J.K. Buitelaar, Prenatal maternal cortisol levels and infant behavior during the first 5 months, Early Hum. Dev. 74 (2003) 139 – 151. [56] S.W. Derbyshire, Locating the beginnings of pain, Bioethics 13 (1999) 1 – 31. [57] J.P. Desborough, The stress response to trauma and surgery, Br. J. Anaesth. 85 (2000) 109 – 117. [58] M. Dodic, V. Hantzis, J. Duncan, S. Rees, I. Koukoulas, K. Johnson, E.M. Wintour, K. Moritz, Programming effects of short prenatal exposure to cortisol, FASEB J. 16 (2002) 1017 – 1026. [59] M. Dolling, R.F. Seamark, Progesterone metabolites in fetal sheep plasma: the effect of nephrectomy, J. Dev. Physiol. 1 (1979) 399 – 413. [60] A. Donaldson, U. Nicolini, E.K. Symes, C.H. Rodeck, Y. Tannirandron, Changes in concentrations of cortisol, dehydroepiandrosterone sulphate and progesterone in fetal and maternal serum during pregnancy, Clin. Endocrinol. 35 (1991) 447 – 451. [61] T.V. Dunwiddie, S.A. Masino, The role and regulation of adenosine in the central nervous system, Annu. Rev. Neurosci. 24 (2001) 31 – 55. [62] F.A. Eales, J. Small, Summit metabolism in newborn lambs, Res. Vet. Sci. 29 (1980) 211 – 218. [63] B.M. Evans, Sleep, consciousness and the spontaneous and evoked electrical activity of the brain. Is there a cortical integrating mechanism? Neurophysiol. Clin. 33 (2003) 1 – 10. [64] J.A. Eyre, S. Miller, G.J. Clowry, E.A. Conway, C. Watts, Functional corticospinal projections are established prenatally in the human foetus permitting involvement in the development of spinal motor centres, Brain 123 (Pt. 1) (2000) 51 – 64. [65] R. Ferri, R. Chiaramonti, M. Elia, S.A. Musumeci, A. Ragazzoni, C.J. Stam, Nonlinear EEG analysis during sleep in premature and full-term newborns, Clin. Neurophysiol. 114 (2003) 1176 – 1180. [66] M. Fitzgerald, S. Beggs, The neurobiology of pain: developmental aspects, Neuroscientist 7 (2001) 246 – 257. [67] M. Fitzgerald, M. Koltzenburg, The functional development of descending inhibitory pathways in the dorsolateral funiculus of the newborn rat spinal cord, Brain Res. 389 (1986) 261 – 270. [68] R.D. Fitzgerald, C. Hieber, E. Schweitzer, A. Luo, W. Oczenski, F.X. Lackner, Intraoperative catecholamine release in brain-dead organ donors is not suppressed by administration of fentanyl, Eur. J. Anaesthesiol. 20 (2003) 952 – 956. [69] H. Foo, P. Mason, Brainstem modulation of pain during sleep and waking, Sleep Med. Rev. 7 (2003) 145 – 154. [70] S. George, A.J. Gunn, J.A. Westgate, C. Brabyn, J. Guan, L. Bennet, [71] [72] [73] [74] [75] [76] [77] [78] [79] [80] [81] [82] [83] [84] [85] [86] [87] [88] [89] [90] [91] 13 Fetal heart rate variability and brainstem injury after asphyxia in preterm fetal sheep, Am. J. Physiol. Regul., Integr. Comp. Physiol. 287 (4) (2004) R925 – R933. X. Giannakoulopoulos, W. Sepulveda, P. Kourtis, V. Glover, N.M. Fisk, Fetal plasma cortisol and beta-endorphin response to intrauterine needling, Lancet 344 (1994) 77 – 81. X. Giannakoulopoulos, J. Teixeira, N. Fisk, V. Glover, Human fetal and maternal noradrenaline responses to invasive procedures, Pediatr. Res. 45 (1999) 494 – 499. J.L. Gingras, K.J. O’Donnell, State control in the substance-exposed fetus: I. The fetal neurobehavioral profile: an assessment of fetal state, arousal, and regulation competency, Ann. N.Y. Acad. Sci. 846 (1998) 262 – 276. R. Gitau, N.M. Fisk, J.M. Teixeira, A. Cameron, V. Glover, Fetal hypothalamic–pituitary–adrenal stress responses to invasive procedures are independent of maternal responses, J. Clin. Endocrinol. Metab. 86 (2001) 104 – 109. R. Gitau, N.M. Fisk, V. Glover, Human fetal and maternal corticotrophin releasing hormone responses to acute stress, Arch. Dis. Child., Fetal Neonatal Ed. 89 (2004) F29 – F32. D.A. Giussani, J.A. Spencer, P.J. Moore, L. Bennet, M.A. Hanson, Afferent and efferent components of the cardiovascular reflex responses to acute hypoxia in term fetal sheep, J. Physiol. 461 (1993) 431 – 449. D.A. Giussani, H.H. McGarrigle, P.J. Moore, L. Bennet, J.A. Spencer, M.A. Hanson, Carotid sinus nerve section and the increase in plasma cortisol during acute hypoxia in fetal sheep, J. Physiol. 477 (Pt. 1) (1994) 75 – 80. V. Glover, N.M. Fisk, Fetal pain: implications for research and practice, Br. J. Obstet. Gynaecol. 106 (1999) 881 – 886. P.D. Gluckman, B.M. Johnston, Lesions in the upper lateral pons abolish the hypoxic depression of breathing in unanaesthetized fetal lambs in utero, J. Physiol. 382 (1987) 373 – 383. P.D. Gluckman, T.R. Gunn, B.M. Johnston, The effect of cooling on breathing and shivering in unanaesthetised fetal lambs in utero, J. Physiol. 343 (1993) 495 – 506. M.S. Golub, Labor analgesia and infant brain development, Pharmacol. Biochem. Behav. 55 (1996) 619 – 628. C. Gottesmann, The transition from slow-wave sleep to paradoxical sleep: evolving facts and concepts of the neurophysiological processes underlying the intermediate stage of sleep, Neurosci. Biobehav. Rev. 20 (1996) 367 – 387. C. Gottesmann, Brain inhibitory mechanisms involved in basic and higher integrated sleep processes, Brain Res. Brain Res. Rev. 45 (2004) 230 – 249. A. Gottschalk, C.L. Wu, E.A. Ochroch, Current treatment options for acute pain, Expert Opin. Pharmacother. 3 (2002) 1599 – 1611. J.A. Grass, The role of epidural anesthesia and analgesia in postoperative outcome, Anesthesiol. Clin. North Am. 18 (2000) 407 – 428. W.C. Griffin III, H.D. Skinner, A.K. Salm, D.L. Birkle, Mild prenatal stress in rats is associated with enhanced conditioned fear, Physiol. Behav. 79 (2003) 209 – 215. R. Grunau, Early pain in preterm infants. A model of long-term effects, Clin. Perinatol. 29 (2002) 373 – 394. T.R. Gunn, B.M. Johnston, H.S. Iwamoto, M. Fraser, M.G. Nicholls, P.D. Gluckman, Haemodynamic and catecholamine responses to hypothermia in the fetal sheep in utero, J. Dev. Physiol. 7 (1985) 241 – 249. T.R. Gunn, J. Butler, P.D. Gluckman, Metabolic and hormonal responses to cooling the fetal sheep in utero, J. Dev. Physiol. 8 (1986) 55 – 66. T.R. Gunn, K.T. Ball, P.D. Gluckman, Reversible umbilical cord occlusion: effects on thermogenesis in utero, Pediatr. Res. 30 (1991) 513 – 517. R. Guzman-Marin, M.N. Alam, R. Szymusiak, R. Drucker-Colin, H. Gong, D. McGinty, Discharge modulation of rat dorsal raphe ARTICLE IN PRESS 14 [92] [93] [94] [95] [96] [97] [98] [99] [100] [101] [102] [103] [104] [105] [106] [107] [108] [109] [110] [111] [112] D.J. Mellor et al. / Brain Research Reviews xx (2005) xxx–xxx neurons during sleep and waking: effects of preoptic/basal forebrain warming, Brain Res. 875 (2000) 23 – 34. J.H.a.T. Hahn, B.R, Neonatal and pediatric electroencephalography, in: M.J. Aminoff (Ed.), Electrodiagnosis in clinical neurology, Churchill Livingstone, Philidelphia, 1999, pp. 81 – 127. R. Harding, E.R. Poore, G.L. Cohen, The effect of brief episodes of diminished uterine blood flow on breathing movements, sleep states and heart rate in fetal sheep, J. Dev. Physiol. 3 (1981) 231 – 243. M.R. Harrison, Surgically corrected fetal disease, Am. J. Surg. 180 (2000) 335 – 342. S.U. Hasan, A. Rigaux, The effects of lung distension, oxygenation, and gestational age on fetal behavior and breathing movements in sheep, Pediatr. Res. 30 (1991) 193 – 201. O. Hayaishi, Invited review: molecular genetic studies on sleep– wake regulation, with special emphasis on the prostaglandin D2 system, J. Appl. Physiol. 92 (2002) 863 – 868. O. Hayaishi, Y. Urade, Prostaglandin D2 in sleep–wake regulation: recent progress and perspectives, Neuroscientist 8 (2001) 12 – 15. T.C. Hays, R. Szymusiak, D. McGinty, GABAA receptor modulation of temperature sensitive neurons in the diagonal band of Broca in vitro, Brain Res. 845 (1999) 215 – 223. P. Hercz, L. Ungar, P. Siklos, Perinatal progesterone in maternal– fetoplacental system during mature and premature deliveries, Acta Obstet. Gynecol. Scand. 67 (1988) 233 – 235. E. Herlenius, H. Lagercrantz, Neurotransmitters and neuromodulators during early human development, Early Hum. Dev. 65 (2001) 21 – 37. M. Hill, A. Parizek, M. Bicikova, H. Havlikova, J. Klak, T. Fait, D. Cibula, R. Hampl, A. Cegan, J. Sulcova, L. Starka, Neuroactive steroids, their precursors, and polar conjugates during parturition and postpartum in maternal and umbilical blood: I. Identification and simultaneous determination of pregnanolone isomers, J. Steroid Biochem. 75 (2000) 237 – 244. J.J. Hirst, K. Egodagamage, D.W. Walker, Effect of a neuroactive steroid infused into the cerebral ventricles of fetal sheep in utero using small infusion volumes, J. Neurosci. Methods 97 (2000) 37 – 44. S.B. Hooper, R. Harding, J. Deayton, G.D. Thorburn, Role of prostaglandins in the metabolic responses of the fetus to hypoxia, Am. J. Obstet. Gynecol. 166 (1992) 1568 – 1575. J.A. Horne, REM sleep—by default? Neurosci. Biobehav. Rev. 24 (2000) 777 – 797. J.A. Horne, A.J. Reid, Night-time sleep EEG changes following body heating in a warm bath, Electroencephalogr. Clin. Neurophysiol. 60 (1985) 154 – 157. S. Hu, W.S. Sheng, J.R. Lokensgard, P.K. Peterson, Morphine induces apoptosis of human microglia and neurons, Neuropharmacology 42 (2002) 829 – 836. C.J. Hunter, L. Bennet, G.G. Power, V. Roelfsema, A.B. Blood, J.S. Quaedackers, S. George, J. Guan, A.J. Gunn, Key neuroprotective role for endogenous adenosine A1 receptor activation during asphyxia in the fetal sheep, Stroke 34 (2003) 2240 – 2245. M.A. Hyatt, D.A. Walker, T. Stephenson, M.E. Symonds, Ontogeny and nutritional manipulation of the hepatic prolactin-growth hormone-insulin-like growth factor axis in the ovine fetus and in neonate and juvenile sheep, Proc. Nutr. Soc. 63 (2004) 127 – 135. S. Ioffe, A.H. Jansen, B.J. Russell, V. Chernick, Sleep, wakefulness and the monosynaptic reflex in fetal and newborn lambs, Pflugers Arch. 388 (1980) 149 – 157. A.H. Jansen, V. Chernick, Fetal breathing and development of control of breathing, J. Appl. Physiol. 70 (1991) 1431 – 1446. G. Jenkin, G. Jorgensen, J.E. Buster, G.D. Thorburn, P.W. Nathanielsz, Induction of premature delivery in sheep following cortisol to the fetus: I. The effect of maternal administration of progestagens, Can. J. Physiol. Pharmacol. 63 (1985) 500 – 508. A. Jensen, Y. Garnier, R. Berger, Dynamics of fetal circulatory responses to hypoxia and asphyxia, Eur. J. Obstet. Gynecol. Reprod. Biol. 84 (1999) 155 – 172. [113] B.M. Johnston, P.D. Gluckman, GABA-mediated inhibition of breathing in the late gestation sheep fetus, J. Dev. Physiol. 5 (1983) 353 – 360. [114] N. Kawai, S. Morokuma, M. Tomonaga, N. Horimoto, M. Tanaka, Associative learning and memory in a chimpanzee fetus: learning and long-lasting memory before birth, Dev. Psychobiol. 44 (2004) 116 – 122. [115] T. Kiserud, E. Jauniaux, D. West, O. Ozturk, M.A. Hanson, Circulatory responses to maternal hyperoxaemia and hypoxaemia assessed non-invasively in fetal sheep at 0.3–0.5 gestation in acute experiments, BJOG 108 (2001) 359 – 364. [116] M.A. Kisley, S.D. Polk, R.G. Ross, P.M. Levisohn, R. Freedman, Early postnatal development of sensory gating, NeuroReport 14 (2003) 693 – 697. [117] S. Kitamura, I. Yokota, H. Hosoda, Y. Kotani, J. Matsuda, E. Naito, M. Ito, K. Kangawa, Y. Kuroda, Ghrelin concentration in cord and neonatal blood: relation to fetal growth and energy balance, J. Clin. Endocrinol. Metab. 88 (2003) 5473 – 5477. [118] V.J. Klimach, R.W. Cooke, Maturation of the neonatal somatosensory evoked response in preterm infants, Dev. Med. Child. Neurol. 30 (1988) 208 – 214. [119] A. Klopper, F. Fuchs, Progestagens, in: F. Fuchs, A. Klopper (Eds.), Endocrinology of pregnancy, Harper and Law Publishers, Maryland, USA, 1977, pp. 99 – 122. [120] B.J. Koos, W. Doany, Role of plasma adenosine in breathing responses to hypoxia in fetal sheep, J. Dev. Physiol. 16 (1991) 81 – 85. [121] B.J. Koos, K. Matsuda, Fetal breathing, sleep state and cardiovascular responses to adenosine in sheep, J. Appl. Physiol. 68 (1990) 489 – 495. [122] B.J. Koos, T. Maeda, C. Jan, Adenosine A1 and A2A receptors modulate sleep state and breathing in fetal sheep, J. Appl. Physiol. 91 (2001) 343 – 350. [123] K. Kubonoya, G.G. Power, Plasma adenosine responses during repeated episodes of umbilical cord occlusion, Am. J. Obstet. Gynecol. 177 (1997) 395 – 401. [124] K.M. Kuczkowski, Severe persistent fetal bradycardia following subarachnoid administration of fentanyl and bupivacaine for induction of a combined spinal–epidural analgesia for labor pain, J. Clin. Anesth. 16 (2004) 78 – 79. [125] I.M. Kuipers, W.J. Maertzdorf, H. Keunen, D.S. De Jong, M.A. Hanson, C.E. Blanco, Fetal breathing is not initiated after cord occlusion in the unanaesthetized fetal lamb in utero, J. Dev. Physiol. 17 (1992) 233 – 240. [126] I.M. Kuipers, W.J. Maertzdorf, D.S. De Jong, M.A. Hanson, C.E. Blanco, The effect of hypercapnia and hypercapnia associated with central cooling on breathing in unanesthetized fetal lambs, Pediatr. Res. 41 (1997) 90 – 95. [127] H. Lagercrantz, Stress, arousal, and gene activation at birth, News Physiol. Sci. 11 (1996) 214 – 218. [128] H. Lagercrantz, T.A. Slotkin, The bstressQ of being born, Sci. Am. 254 (1986) 92 – 102. [129] W.R. Lariviere, R. Melzack, The role of corticotropin-releasing factor in pain and analgesia, Pain 84 (2000) 1 – 12. [130] G. Le Masson, S. Renaud-Le Masson, D. Debay, T. Bal, Feedback inhibition controls spike transfer in hybrid thalamic circuits, Nature 417 (2002) 854 – 858. [131] L.R. Leader, A.D. Stevens, E.R. Lumbers, Measurement of fetal responses to vibroacoustic stimuli. Habituation in fetal sheep, Biol. Neonate 53 (1988) 73 – 85. [132] B. Lee, J.J. Hirst, D.W. Walker, Prostaglandin D synthase in the prenatal ovine brain and effects of its inhibition with selenium chloride on fetal sleep/wake activity in utero, J. Neurosci. 22 (2002) 5679 – 5686. [133] L. Lehtonen, R.J. Martin, Ontogeny of sleep and awake states in relation to breathing in preterm infants, Semin. Neonatol. 9 (2004) 229 – 238. [134] E.D. Lephart, Brain 5alpha-reductase: cellular, enzymatic, and ARTICLE IN PRESS D.J. Mellor et al. / Brain Research Reviews xx (2005) xxx–xxx [135] [136] [137] [138] [139] [140] [141] [142] [143] [144] [145] [146] [147] [148] [149] [150] [151] [152] [153] [154] [155] [156] molecular perspectives and implications for biological function, Mol. Cell. Neurosci. 4 (1993) 473 – 484. A.S. Lijowska, N.W. Reed, B.A. Chiodini, B.T. Thach, Sequential arousal and airway-defensive behavior of infants in asphyxial sleep environments, J. Appl. Physiol. 83 (1997) 219 – 228. A.R. Lloyd-Thomas, M. Fitzgerald, Do fetuses feel pain? Reflex responses do not necessarily signify pain, BMJ 313 (1996) 797 – 798. K. Lopau, J. Mark, L. Schramm, E. Heidbreder, C. Wanner, Hormonal changes in brain death and immune activation in the donor, Transplant. Int. 1 (2000) S282 – S285. M.H. Maguire, T.P. Krishnakantha, AMP phosphatase activity in human term placenta: studies on placental 5V-nucleotidase, in: I.s.o.p.m.i. Man (Ed.), Purine metabolism in man, vol. 3, Plenum, Madrid, 1980, pp. 393 – 396. M.D. Majewska, Neurosteroids: endogenous bimodal modulators of the GABAA receptor. Mechanism of action and physiological significance, Progr. Neurobiol. 38 (1992) 379 – 395. R. Marana, F. Margutti, G.F. Catalano, E. Marana, Stress responses to endoscopic surgery, Curr. Opin. Obstet. Gynecol. 12 (2000) 303 – 307. C. Mardirosoff, L. Dumont, M. Boulvain, M.R. Tramer, Fetal bradycardia due to intrathecal opioids for labour analgesia: a systematic review, BJOG 109 (2002) 274 – 281. G.A. Marks, J.P. Shaffery, A. Oksenberg, S.G. Speciale, H.P. Roffwarg, A functional role for REM sleep in brain maturation, Behav. Brain Res. 69 (1995) 1 – 11. K. Marsal, Ultrasonic assessment of fetal activity, Clin. Obstet. Gynaecol. 10 (1983) 541 – 563. G. Mastorakos, I. Ilias, Maternal and fetal hypothalamic–pituitary– adrenal axes during pregnancy and postpartum, Ann. N.Y. Acad. Sci. 997 (2003) 136 – 149. H. Matsumura, T. Nakajima, T. Osaka, S. Satoh, K. Kawase, E. Kubo, S.S. Kantha, K. Kasahara, O. Hayaishi, Prostaglandin D2sensitive, sleep-promoting zone defined in the ventral surface of the rostral basal forebrain, Proc. Natl. Acad. Sci. U. S. A. 91 (1994) 11998 – 12002. D.A. McCormick, T. Bal, Sleep and arousal: thalamocortical mechanisms, Annu. Rev. Neurosci. 20 (1997) 185 – 215. D. McGinty, R. Szymusiak, Brain structures and mechanisms involved in the generation of NREM sleep: focus on the preoptic hypothalamus, Sleep Med. Rev. 5 (2001) 323 – 342. G.H. McIntosh, K.I. Baghurst, B.J. Potter, B.S. Hetzel, Foetal brain development in the sheep, Neuropathol. Appl. Neurobiol. 5 (1979) 103 – 114. F. McNamara, H. Wulbrand, B.T. Thach, Characteristics of the infant arousal response, J. Appl. Physiol. 85 (1998) 2314 – 2321. F. McNamara, A.S. Lijowska, B.T. Thach, Spontaneous arousal activity in infants during NREM and REM sleep, J. Physiol. 538 (2002) 263 – 269. D.J. Mellor, N.G. Gregory, Responsiveness, behavioural arousal and awareness in fetal and newborn lambs: experimental, practical and therapeutic implications, N. Z. Vet. J. 51 (2003) 2 – 13. R. Melzack, T.J. Coderre, J. Katz, A.L. Vaccarino, Central neuroplasticity and pathological pain, Ann. N. Y. Acad. Sci. 933 (2001) 157 – 174. R.M. Mentzer, S.W. Ely, R.D. Lasley, R.D. Mainwaring, E.M. Wright, R.M. Berne, Hormonal role of adenosine in maintaining patency of the ductus arteriosus in fetal lambs, Ann. Surg. 202 (1985) 223 – 230. M.M. Methippara, M.N. Alam, R. Szymusiak, D. McGinty, Preoptic area warming inhibits wake-active neurons in the perifornical lateral hypothalamus, Brain Res. 960 (2003) 165 – 173. H. Mickan, J. Zander, Preganolones, pregnenolone and progesterone in the human feto-placental circulation at term of pregnancy, J. Steroid Biochem. 11 (1979) 1461 – 1466. W.L. Miller, Steroid hormone biosynthesis and actions in the materno-feto-placental unit, Clin. Perinatol. 25 (1998) 799 – 817. 15 [157] M. Mirmiran, Y.G. Maas, R.L. Ariagno, Development of fetal and neonatal sleep and circadian rhythms, Sleep Med. Rev. 7 (2003) 321 – 334. [158] S. Moiniche, H. Kehlet, J.B. Dahl, A qualitative and quantitative systematic review of preemptive analgesia for postoperative pain relief: the role of timing of analgesia, Anesthesiology 96 (2002) 725 – 741. [159] G.H. Moser, J. Schrader, A. Deussen, Turnover of adenosine in plasma of human and dog blood, Am. J. Physiol. 256 (1989) C799 – C806. [160] L. Nardo, Y. Soong, D. Wu, I.R. Young, D. Walker, H.H. Szeto, Site and mechanism of action of dynorphin A-(1–13) and N-methyl-daspartate on ACTH release in fetal sheep, Am. J. Physiol. Endocrinol. Metab. 282 (2002) E1301 – E1307. [161] P. Naveilhan, J.M. Canals, E. Arenas, P. Ernfors, Distinct roles of the Y1 and Y2 receptors on neuropeptide Y-induced sensitization to sedation, J. Neurochem. 78 (2001) 1201 – 1207. [162] I.D. Neumann, A. Wigger, G. Liebsch, F. Holsboer, R. Landgraf, Increased basal activity of the hypothalamo–pituitary–adrenal axis during pregnancy in rats bred for high anxiety-related behaviour, Psychoneuroendocrinology 23 (1998) 449 – 463. [163] P.N. Nguyen, S.S. Billiards, D.D. Walker, J.J. Hirst, Changes in 5 alpha-pregnane steroids and neurosteroidogenic enzyme expression in the perinatal sheep, Pediatr. Res. 53 (2003) 956 – 964. [164] M.B. Nicol, J.J. Hirst, D. Walker, G.D. Thorburn, Effect of alteration of maternal plasma progesterone concentrations on fetal behavioural state during late gestation, J. Endocrinol. 152 (1997) 379 – 386. [165] M.B. Nicol, J.J. Hirst, D.W. Walker, Effect of pregnane steroids on electrocortical activity and somatosensory evoked potentials, Neurosci. Lett. 253 (1998) 111 – 114. [166] M.B. Nicol, J.J. Hirst, D. Walker, Effects of pregnanolone on behavioural parameters and the responses to GABAA receptor antagonists in the late gestation fetal sheep, Neuropharmacology 38 (1999) 49 – 63. [167] M.B. Nicol, J.J. Hirst, D.W. Walker, Effect of finasteride on behavioural arousal and somatosensory evoked potentials in fetal sheep, Neurosci. Lett. 306 (2001) 13 – 16. [168] J.G. Nijhuis, Fetal behavior, Neurobiol. Aging 24 (Suppl. 1) (2003) S41 – S46 (discussion S47–S49, S51–S42). [169] F.J. Obal, J.M. Krueger, Biochemical regulation of non-rapid-eyemovement sleep, Front. Biosci. 8 (2003) d520 – d550. [170] T. Okai, S. Kozuma, N. Shinozuka, Y. Kuwabara, M. Mizuno, A study on the development of sleep-wakefulness cycle in the human fetus, Early Hum. Dev. 29 (1992) 391 – 396. [171] G.D. Olsen, T.M. Cline, K.M. Sommer, Comparison of chronic morphine and placebo infusion in late gestation fetal lambs: effect upon survival and breathing movements, J. Pharmacol. Exp. Ther. 247 (1988) 162 – 168. [172] M.J. Parkes, Fetal behavioural states: sleep and wakefulness? Q. J. Exp. Psychobiol. 44B (1992) 231 – 244. [173] P.M. Parslow, R. Harding, T.M. Adamson, R.S. Horne, Of sleep state and postnatal age on arousal responses induced by mild hypoxia in infants, Sleep 27 (2004) 105 – 109. [174] D. Pattinson, M. Fitzgerald, The neurobiology of infant pain: development of excitatory and inhibitory neurotransmission in the spinal dorsal horn, Reg. Anesth. Pain Med. 29 (2004) 36 – 44. [175] S.M. Paul, R.H. Purdy, Neuroactive steroids, FASEB J. 6 (1992) 2311 – 2322. [176] M.A. Perez-Pinzon, G.E. Nilsson, P.L. Lutz, Relationship between ion gradients and neurotransmitter release in the newborn rat striatum during anoxia, Brain Res. 602 (1993) 228 – 233. [177] R.G. Peterson, Consequences associated with nonnarcotic analgesics in the fetus and newborn, Fed. Proc. 44 (1985) 2309 – 2313. [178] S. Petratos, J.J. Hirst, S. Mendis, P. Anikijenko, D.W. Walker, Localization of P450scc and 5alpha-reductase type-2 in the cerebellum of fetal and newborn sheep, Dev. Brain Res. 123 (2000) 81 – 86. ARTICLE IN PRESS 16 D.J. Mellor et al. / Brain Research Reviews xx (2005) xxx–xxx [179] M. Pilon, S.J. Sullivan, Motor profile of patients in minimally responsive and persistent vegetative states, Brain Inj. 10 (1996) 421 – 437. [180] T. Porkka-Heiskanen, L. Alanko, A. Kalinchuk, D. Stenberg, Adenosine and sleep, Sleep Med. Rev. 6 (2002) 321 – 332. [181] G.G. Power, Biology of temperature: the mammalian fetus, J. Dev. Physiol. 12 (1989) 295 – 304. [182] H.F. Prechtl, Prenatal development of postnatal behavior, In: H. Rauh, H.C. Steinhausen (Eds.), Psychobiology and early development, Elsevier, Amsterdam, 1987, pp. 231 – 238. [183] H.F. Prechtl, J.G. Nijhuis, Eye movements in the human fetus and newborn, Behav. Brain Res. 10 (1983) 119 – 124. [184] K. Ptak, E. Di Pasquale, R. Monteau, Substance P and central respiratory activity: a comparative in vitro study on foetal and newborn rat, Brain Res. Dev. Brain Res. 114 (1999) 217 – 227. [185] K. Rees, D.P. Spence, J.E. Earis, P.M. Calverley, Arousal responses from apneic events during non-rapid-eye-movement sleep, Am. J. Respir. Crit. Care Med. 152 (1995) 1016 – 1021. [186] B.S. Richardson, The effect of behavioral state on fetal metabolism and blood flow circulation, Semin. Perinatol. 16 (1992) 227 – 233. [187] B.S. Richardson, A.D. Bocking, Metabolic and circulatory adaptations to chronic hypoxia in the fetus, Comp. Biochem. Physiol., A. Mol. Integr. Physiol. 119 (1998) 717 – 723. [188] H. Rigatto, Control of breathing in fetal life and onset and control of breathing in the neonate, In: R.A. Polin, W.W. Fox, S.H. Abman (Eds.), Fetal and neonatal physiology, vol. 1, Saunders, Philadelphia, 2004, pp. 890 – 900. [189] H. Rigatto, C.E. Blanco, D.W. Walker, The response to stimulation of hindlimb nerves in fetal sheep, in utero, during the different phases of electrocortical activity, J. Dev. Physiol. 4 (1982) 175 – 185. [190] H. Rigatto, M. Moore, D. Cates, Fetal breathing and behavior measured through a double-wall Plexiglas window in sheep, J. Appl. Physiol. 61 (1986) 160 – 164. [191] H. Rigatto, D. Lee, M. Davi, M. Moore, E. Rigatto, D. Cates, Effect of increased arterial CO2 on fetal breathing and behavior in sheep, J. Appl. Physiol. 64 (1988) 982 – 987. [192] H.P. Roffwarg, J.N. Munzio, W.C. Dement, Ontogenetic development of the human sleep–dream cycle, Science 152 (1966) 604 – 619. [193] Y. Ruckebusch, Development of sleep and wakefulness in the foetal lamb, Electroecephalogr. Clin. Neurophysiol. 32 (1972) 119 – 128. [194] Y. Ruckebusch, M. Gaujoux, B. Eghbali, Sleep cycles and kinesis in the fetal lamb, Electroecephalogr. Clin. europhysiol. 42 (1977) 226 – 237. [195] D.W. Rurak, M.R. Wright, J.E. Axelson, Drug disposition and effects in the fetus, J. Dev. Physiol. 15 (1991) 33 – 44. [196] R.I.R. Russell, A. Greenough, H. Lagercrantz, I. Dahlin, K. Nicolaides, Fetal anaemia and its relation with increased concentrations of adenosine, Arch. Dis. Child. 68 (1993) 35 – 36. [197] H. Saitoh, K. Hirato, T. Yanaihara, T. Nakayama, A study of 5 alphareductase in human fetal brain, Endocrinol. Jpn. 29 (1982) 461 – 467. [198] C.A. Sandman, P. Wadhwa, L. Glynn, A. Chicz-Demet, M. Porto, T.J. Garite, Corticotrophin-releasing hormone and fetal responses in human pregnancy, Ann. N. Y. Acad. Sci. 897 (1999) 66 – 75. [199] E.M. Sanhueza, A.A. Johansen-Bibby, A.J. Fletcher, R.A. Riquelme, A.J. Daniels, M. Seron-Ferre, C.R. Gaete, J.E. Carrasco, A.J. Llanos, D.A. Giussani, The role of neuropeptide Y in the ovine fetal cardiovascular response to reduced oxygenation, J. Physiol. 546 (2003) 891 – 901. [200] R. Sawa, H. Asakura, G.G. Power, Changes in plasma adenosine during stimulated birth of fetal sheep, J. Appl. Physiol. 70 (1991) 1524 – 1528. [201] A. Scharf, M. Seppelt, C. Sohn, Doppler flow velocity to measure the redistribution of fetal cardiac output in fetal stress, Eur. J. Obstet. Gynecol. Reprod. Biol. 110 (Suppl. 1) (2003) S119 – S126. [202] M.S. Scher, Pediatric neurophysiologic evaluation, In: K.F. Swaiman, S. Ashwal (Eds.), Pediatric neurology, Principles and practice, vol. 1, Mosby, St Louis, 1999, pp. 142 – 181. [203] M. Schwab, T. Bludau, R.M. Abrams, P.J. Antonelli, K.J. Gerhardt, R. Bauer, Thermal stimulation of the fetal skin induces ECoG arousal in fetal sheep, Pflugers Arch.: Eur. J. Phsyiol. 433 (1997) P594. [204] M. Schwab, K. Schmidt, H. Witte, M. Abrams, Investigation of nonlinear ECoG changes during spontaneous sleep state changes and cortical arousal in fetal sheep, Cereb. Cortex 10 (2000) 142 – 148. [205] R.F. Seamark, C.D. Nancarrow, J. Gardner, Progesterone metabolism in ovine fetal blood: the formation of 3alpha-hydroxy-pregn-4-en-20one and other substances, Steroids 15 (1970) 589 – 603. [206] H.J. Shelley, Glycogen reserves and their changes at birth and in anoxia, Br. Med. Bull. 17 (1961) 137 – 143. [207] A. Siddiqui, S. Haq, B.H. Shah, Perinatal exposure to morphine disrupts brain norepinephrine, ovarian cyclicity, and sexual receptivity in rats, Pharmacol. Biochem. Behav. 58 (1997) 243 – 248. [208] S.H. Simons, M. van Dijk, R.A. van Lingen, D. Roofthooft, H.J. Duivenvoorden, N. Jongeneel, C. Bunkers, E. Smink, K.J. Anand, J.N. van den Anker, D. Tibboel, Routine morphine infusion in preterm newborns who received ventilatory support: a randomized controlled trial, JAMA 290 (2003) 2419 – 2427. [209] P. Slegel, H. Kitagawa, H. Maguire, Determination of adenosine in fetal perfusates of human placental cotyledons using fluorescence derivatization and reversed-phase high-performance liquid chromatography, Anal. Biochem. 171 (1988) 124 – 134. [210] R.P. Smith, R. Gitau, V. Glover, N.M. Fisk, Pain and stress in the human fetus, Eur. J. Obstet. Gynecol. Reprod. Biol. 92 (2000) 161 –165. [211] R.P. Smith, V. Glover, N.M. Fisk, Acute increase in femoral artery resistance in response to direct physical stimuli in the human fetus, BJOG 110 (2003) 916 – 921. [212] R.P. Smith, S.L. Miller, N. Igosheva, D.M. Peebles, V. Glover, G. Jenkin, M.A. Hanson, N.M. Fisk, Cardiovascular and endocrine responses to cutaneous electrical stimulation after fentanyl in the ovine fetus, Am. J. Obstet. Gynecol. 190 (2004) 836 – 842. [213] W.P. Smotherman, C.A. Moody, L.P. Spear, S.R. Robinson, Fetal behavior and the endogenous opioid system: D1 dopamine receptor interactions with the kappa opioid system, Physiol. Behav. 53 (1993) 191 – 197. [214] E. Sollevi, Cardiovascular effects of adenosine in man; possible clinical implications, Progr. Neurobiol. 27 (1986) 319 – 349. [215] J.A. Spencer, Predictive value of a fetal heart rate acceleration at the time of fetal blood sampling in labour, J. Perinat. Med. 19 (1991) 207 – 215. [216] R. Stickgold, J.A. Hobson, R. Fosse, M. Fosse, Sleep, learning, and dreams: off-line memory reprocessing, Science 294 (2001) 1052 – 1057. [217] A. Stiene-Martin, P.E. Knapp, K. Martin, J.A. Gurwell, S. Ryan, S.R. Thornton, F.L. Smith, K.F. Hauser, Opioid system diversity in developing neurons, astroglia, and oligodendroglia in the subventricular zone and striatum: impact on gliogenesis in vivo, Glia 36 (2001) 78 – 88. [218] N. Suntsova, R. Szymusiak, M.N. Alam, R. Guzman-Marin, D. McGinty, Sleep-waking discharge patterns of median preoptic nucleus neurons in rats, J. Physiol. 543 (2002) 665 – 677. [219] H.H. Szeto, Behavioral states and their ontogeny: animal studies, Semin. Perinatol. 16 (1992) 211 – 216. [220] H.H. Szeto, D.J. Hinman, Prenatal development of sleep–wake patterns in sheep, Sleep 8 (1985) 347 – 355. [221] H.H. Szeto, J.G. Umans, The effects of a stable adenosine analogue on fetal behavioural, respiratory and cardiovascular functions, in: C.T. Jones, P.W. Nathanielsz (Eds.), The physiological development of the fetus and newborn, Academic Press, London, 1985, pp. 649 – 652. [222] H.H. Szeto, Y. Soong, D.L. Wu, P.Y. Cheng, Opioid modulation of fetal glucose homeostasis: role of receptor subtypes, J. Pharmacol. Exp. Ther. 275 (1995) 334 – 339. [223] C.C. Taylor, Y.I. Soong, D. Wu, J.S. Yee, H.H. Szeto, Morphine stimulates adrenocorticotropin and cortisol release in the late-term ovine fetus, Pediatr. Res. 41 (1997) 411 – 415. ARTICLE IN PRESS D.J. Mellor et al. / Brain Research Reviews xx (2005) xxx–xxx [224] J.M. Teixeira, V. Glover, N.M. Fisk, Acute cerebral redistribution in response to invasive procedures in the human fetus, Am. J. Obstet. Gynecol. 181 (1999) 1018 – 1025. [225] B.T. Thach, Graded arousal responses in infants: advantages and disadvantages of a low threshold for arousal, Sleep Med. 3 (Suppl. 2) (2002) S37 – S40. [226] I. Thaler, R. Boldes, I. Timor-Tritsch, Real-time spectral analysis of the fetal EEG: a new approach to monitoring sleep states and fetal condition during labor, Pediatr. Res. 48 (2000) 340 – 345. [227] E. Van Cauter, F. Latta, A. Nedeltcheva, K. Spiegel, R. Leproult, C. Vandenbril, R. Weiss, J. Mockel, J.J. Legros, G. Copinschi, Reciprocal interactions between the GH axis and sleep, Growth Horm. IGF Res. 14 (Suppl. A) (2004) S10 – S17. [228] M. van de Pas, J.G. Nijhuis, H.W. Jongsma, Fetal behaviour in uncomplicated pregnancies after 41 weeks of gestation, Early Hum. Dev. 40 (1994) 29 – 38. [229] A.J. van der Lely, M. Tschop, M.L. Heiman, E. Ghigo, Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin, Endocr. Rev. 25 (2004) 426 – 457. [230] S. Vanhatalo, O. van Nieuwenhuizen, Fetal pain? Brain Dev. 22 (2000) 145 – 150. [231] R.P. Vertes, Memory consolidation in sleep; dream or reality, Neuron 44 (2004) 135 – 148. [232] J.W. Villiger, K.M. Taylor, P.D. Gluckman, Multiple benzodiazapine receptors in the ovine brain: ontogeny, properties, and distribution of 3 H-diazapam binding, Paediatr. Pharmacol. 2 (1982) 179 – 187. [233] G.H.A. Visser, R.N. Laurini, J.I.P. de Vries, D.J. Bekedam, H.F.R. Prechtl, Abnormal motor behaviour in anencephalic fetuses, Early Hum. Dev. 12 (1985) 173 – 182. [234] G.H. Visser, H.H. Mulder, H.P. Wit, E.J. Mulder, H.F. Prechtl, Vibro- [235] [236] [237] [238] [239] [240] [241] [242] [243] [244] 17 acoustic stimulation of the human fetus: effect on behavioural state organization, Early Hum. Dev. 19 (1989) 285 – 296. J.G. Wettstein, B. Earley, J.L. Junien, Central nervous system pharmacology of neuropeptide Y, Pharmacol. Ther. 65 (1995) 397 – 414. M.C. White, A.R. Wolf, Pain and stress in the human fetus, Best Pract. Res. Clin. Anaesthesiol. 18 (2004) 205 – 220. M.F. Whitfield, R.E. Grunau, Behavior, pain perception, and the extremely low-birth weight survivor, Clin. Perinatol. 27 (2000) 363 – 379. Y. Yoneyama, S. Shin, T. Iwasaki, G. Power, T. Araki, Relationship between plasma adenosine concentrations and breathing movements in growth-retarded fetuses, Am. J. Obstet. Gynecol. 171 (1994) 701 – 706. Y. Yoneyama, M. Wakatsuki, R. Sawa, S. Kamoi, H. Takahashi, S. Shin, T. Kawamura, G.G. Power, T. Araki, Plasma adenosine concentration in appropriate-for-gestational-age and small-for-festational-age fetuses, Am. J. Obstet. Gynecol. 170 (1994) 684 – 688. Y. Yoneyama, R. Sawa, S. Suzuki, S. Shin, G.G. Power, T. Araki, The relationship between uterine artery Doppler velocimetry and umbilical venous adenosine levels in pregnancies complicated by preeclampsia, Am. J. Obstet. Gynecol. 174 (1996) 267 – 271. Y. Yoneyama, S. Suzuki, R. Sawa, Y. Kiyokawa, G.G. Power, T. Araki, Plasma adenosine levels and P-selection expression on platelets in preeclampsia, Obstet. Gynecol. 97 (2001) 366 – 370. A. Zeman, Persistent vegetative state, Lancet 350 (1997) 795 – 799. A. Zeman, Consciousness, Brain 124 (2001) 1263 – 1289. J. Zhang, F. Obal Jr., T. Zheng, J. Fang, P. Taishi, J.M. Krueger, Intrapreoptic microinjection of GHRH or its antagonist alters sleep in rats, J. Neurosci. 19 (1999) 2187 – 2194.