Atoms, Molecules and Compounds, OH MY!

Atoms, Molecules and Compounds,
OH MY!
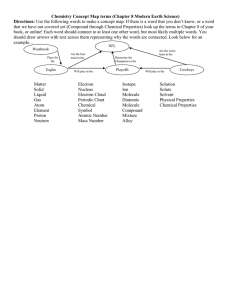
Element
A substance that cannot be chemically split into a simpler substance shares the same set of properties
Examples: Hydrogen, Gold, Neon, Chlorine...
Atom
The smallest unit of an element that can have the chemical properties of that element composed of protons, neutrons and electrons
3057473598700243704305 atoms
Compound
2 or more elements chemically combined ex: H2O, NaOh, CH4...
Molecule
2 or more Atoms chemically combined
Example O2, H2O, CO2, H2, CH4
If the same element is combined called:
Diatomic, ex: O2
Triatomic, ex: O3
But Wait Meltz, that was too easy....
Can a compound be a molecule? Vice Versa?!?
Yes!
A molecule is the smallest unit of a compound that maintains the properties of the compound example:
In 8 ounces of water, there are
7.5x10^24 molecules!
Try This!
An atom is to an element as a _____________ is to a _______________.
Label the following with each word (atom, element, molecule, compound) that applies:
H
H2
H2O