Chapter 12 Review, pages 610–615
advertisement

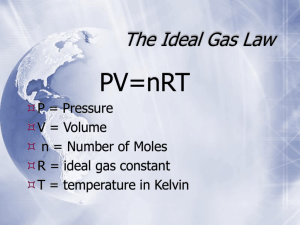
Chapter 12 Review, pages 610–615 Knowledge 1. (b) 2. (c) 3. (d) 4. (b) 5. (d) 6. (b) 7. (a) 8. (b) 9. (c) 10. False. One mole of a gas has a volume of 22.4 L at standard temperature and pressure. 11. True 12. True 13. False. A gas at high temperature behaves more like an ideal gas than does a gas at low temperature. 14. False. Entities in a Bose-Einstein condensate do not behave like individual atoms. 15. True 16. False. When temperature and pressure remain constant during a chemical reaction, Dalton’s law of partial pressure can be used to calculate volumes. 17. True 18. True 19. (a) (iv) (b) (i) (c) (ii) (d) (iii) 20. Gay-Lussac’s observations of chemical reactions involving gases showed that the volumes of gaseous reactants and products are always in whole-number ratios. 21. Avogadro’s law states that the volume of a gas is directly related to the amount of gas when the temperature and pressure stay constant. It also states that equal volumes of gases, under identical conditions, contain the same number of entities. 22. Molar volume at ambient temperature and pressure is greater than molar volume at STP because ambient temperature is considerably greater than temperature at STP and increasing temperature increases the volume of a gas. Ambient pressure and standard pressure are similar, so the difference in pressure only minimally affects molar volume. 23. The entities in an ideal gas move randomly in straight lines in all directions. 24. The statement means that there is no loss of kinetic energy during the collisions between entities. 25. The three gas laws that combine to form the ideal gas law are Charles’ law, Boyle’s law, and Avogadro’s law. 26. In a Bose-Einstein condensate the entities stop behaving like individual atoms and together act more like a single atom. This is a state of matter that occurs only at extremely low temperatures. Other states of matter, in which the entities act more independently, occur at higher temperatures. 27. Absolute zero has not yet been reached, although scientists are getting very close to it. Copyright © 2011 Nelson Education Ltd. Chapter 12: Gas Laws, Gas Mixtures, and Gas Reactions 12-2 28. The total pressure of the air is the sum of the partial pressures: 20.0 kPa + 78.0 kPa + 3.0 kPa = 101 kPa. 29. For Dalton’s law of partial pressures to apply to a mixture of gases, the gases must not react and the units of pressure must be the same. 30. The units of pressure must be the same for Dalton’s law of partial pressures to be used. Pressure units in this statement include both kPa and mm Hg. 31. You can use volume to solve stoichiometry problems involving gases, but not those involving only other states because only gases contain the same number of entities in equal volumes under the same conditions. 32. Temperature and pressure must remain constant when solving stoichiometry problems involving volume because changes in temperature and pressure change the volume of gases. 33. Airbags use a chemical reaction that produces a gas because a gas can fill the airbag quickly enough in a collision to protect a person from injury. 34. It is surprising that methane and water form a gas hydrate together because water is polar and methane is non-polar and usually they do not mix. 35. The environmental problem that might be the result of burning gas hydrates is the release of carbon dioxide, a greenhouse gas, into the atmosphere. 36. A gas hydrate and a compound such as CuSO4•5H2O are both called hydrates because the term hydrate is used when water is added to another compound. Understanding 37. (a) 3 H 2 (g) + N 2 (g) ! 2 NH 3 (b) The ratio of the volume of hydrogen to the volume of nitrogen is 3 : 1. Thus, the volume of nitrogen that reacts with 4.5 L of hydrogen is 1.5 L. (c) The ratio of the volume of hydrogen to the volume of ammonia is 3 : 2. Thus, the volume of NH3 formed from 4.5 L of hydrogen is 3.0 L. 38. Given: initial volume, V1 = 27.3 L initial amount of gas, n1 = 1.5 mol final amount of gas, n2 = n1 + nAr added mass of helium added, mHe added = 2.4 g molar mass of helium added, MHe = 4.00 g/mol The pressure and temperature remain constant. Required: Identify the unknown variable. V2 = ? Analysis: Use Avogadro’s law to find the final volume of the gas. V1 V2 = n1 n2 Solution: Step 1. Determine the amount of helium added, nHe added. 1 mol nHe added = mHe added ! 4.00 g 1 mol = 2.4 g ! 4.00 g nHe added = 0.60 mol Copyright © 2011 Nelson Education Ltd. Chapter 12: Gas Laws, Gas Mixtures, and Gas Reactions 12-3 Step 2. Determine the final total amount of helium, n2. n2 = n1 + nAr added = 1.5 mol + 0.60 mol n2 = 2.1 mol Step 3. Rearrange the Avogadro’s law equation to isolate the unknown variable, substitute in the known values, and solve the equation. Vn V2 = 1 2 n1 = 27.3 L ! 2.1 mol 1.5 mol V2 = 38 L Statement: The new volume of the balloon is 38 L. 39. Use a conversion factor derived from the molar volume for a gas at STP to find the volume of the neon gas. 22.4 L 6.4 mol ! = 140 L 1 mol The volume of the neon gas is 140 L. 40. Given: V = 12.3 L; SATP conditions Required: mass of argon, mAr 1 mol Analysis: n = V ! 24.8 L 39.95 g M Ar = 1 mol Solution: Step 1. Determine the amount of argon gas in the sample by multiplying the volume of argon by an appropriate conversion factor derived from the molar volume at SATP. 1 mol n=V ! 24.8 L 1 mol = 12.3 L ! 24.8 L n = 0.496 mol Step 2. Determine the mass of argon gas in the sample by multiplying the amount of argon by an appropriate conversion factor derived from the molar mass of argon. 39.95 g mAr = n ! 1 mol 39.95 g = 0.496 mol ! 1 mol mAr = 19.8 g Statement: The mass of argon gas is 19.8 g. Copyright © 2011 Nelson Education Ltd. Chapter 12: Gas Laws, Gas Mixtures, and Gas Reactions 12-4 41. (a) Answers may vary. Sample answer: Entities of real gases experience intermolecular forces. He, O2, and Cl2 are all non-polar, so any intermolecular forces are based only on weak London dispersion forces. Helium entities are smaller than those of the other gases. This means there are fewer electrons and protons resulting in attractions among helium atoms compared to the other real gases. Less attraction means that the molecules are farther apart from each other, allowing the gas to occupy a larger volume. (b) Answers may vary. Sample answer: Neon is closer in mass to helium than to oxygen or chlorine, so I expect the molar volume of neon to be closer to that of helium. 42. (a) In addition to the measurements required for the ideal gas law, the molar mass of a gas, M, is needed to determine the mass, m, of the gas. m Note that n = . Substitute this expression for n in the ideal gas law equation. M PV = nRT mRT PV = M Now rearrange this equation to solve for the mass, m. PVM m= RT (b) In addition to the measurements required for the ideal gas law, the mass of a gas, m, is needed to determine the molar mass, M, of the gas. m Note that n = . Substitute this expression for n in the ideal gas law equation. M PV = nRT mRT PV = M Now rearrange this equation to solve for the molar mass, M. mRT M= PV (c) In addition to the measurements required for the ideal gas law, the mass of a gas, m, is needed to determine the density, d, of the gas. m m Note that d = , so V = . Substitute this expression for V in the ideal gas law equation. V d PV = nRT Pm = nRT d Now rearrange this equation to solve for the density, d. Pm d= nRT Copyright © 2011 Nelson Education Ltd. Chapter 12: Gas Laws, Gas Mixtures, and Gas Reactions 12-5 43. Given: V = 27 mL t = 24 ºC P = 102 kPa R = 8.314 kPa i L i mol–1 i K–1 Required: amount of hydrogen gas, n Analysis: Use the ideal gas law equation to calculate the amount of gas produced. PV = nRT Solution: Step 1. Convert the temperature value to kelvins and volume from mL to L. T = t + 273 = 24 + 273 T = 297 K V = 27 mL ! 1L 1000 mL V = 0.027 L Step 2. Rearrange the ideal gas law equation to find the amount of gas. PV n= RT Step 3. Substitute known values into the equation and solve. 102 kPa ! 0.027 L n= 8.314 kPa i L imol–1 i K –1 ! 297 K n = 0.0011 mol Statement: The amount of hydrogen gas produced was 1.1 × 10–3 mol. 44. Given: amount of nitrogen gas, n = 2.5 mol R = 8.314 kPa i L i mol–1 i K–1 M N (g) = 28.02 g/mol 2 The conditions are standard temperature and pressure, so T = 273 K and P = 101.325 kPa. Required: density of nitrogen gas, d N 2 To find the density, you first have to determine the mass, m, and the volume, V. Analysis: First use the ideal gas law to determine the volume of the sample. PV = nRT Next, convert 2.5 mol of nitrogen gas to mass. Finally, use the expression d = m/V to calculate the density of the nitrogen gas. Solution: Step 1. Rearrange the ideal gas law equation to isolate the unknown variable, V. nRT V= P Copyright © 2011 Nelson Education Ltd. Chapter 12: Gas Laws, Gas Mixtures, and Gas Reactions 12-6 Step 2. Substitute known values into the equation and solve. –1 V= 2.5 mol ! 8.314 kPa iLi mol i K –1 ! 273 K 101.325 kPa V = 56 L Step 3. Convert amount of N2 into mass of N2 using the appropriate conversion factor involving molar mass. 28.02 g mN = nN ! 2 2 1 mol 28.02 g = 2.5 mol ! 1 mol mN = 7.0 ! 10 g 2 Step 4. Use the relationship d N = m/V to determine the density of N2. 2 m V 7.0 ! 10 g = 56 L = 1.3 g/L dN = 2 dN 2 Statement: The density of pure nitrogen gas at STP is 1.3 g/L. 45. Given: V = 64 mL t = 22 ºC P = 102 kPa R = 8.314 kPa i L i mol–1 i K–1 Required: amount of chlorine gas, n Analysis: Use the ideal gas law equation to calculate the amount of gas released. PV = nRT Solution: Step 1. Convert the temperature value to kelvins and volume from mL to L. T = t + 273 = 22 + 273 T = 295 K V = 64 mL ! 1L 1000 mL V = 0.064 L Step 2. Rearrange the ideal gas law equation to find the amount of gas. PV n= RT Copyright © 2011 Nelson Education Ltd. Chapter 12: Gas Laws, Gas Mixtures, and Gas Reactions 12-7 Step 3. Substitute known values into the equation and solve. 102 kPa ! 0.064 L n= 8.314 kPa i L imol–1 i K –1 ! 295 K n = 0.0027 mol Statement: The amount of chlorine gas released is 2.7 × 10–3 mol. 46. Gases are liquefied when the attractions among entities in the gas are strong enough to pull the entities close together. Intermolecular forces are based on electrostatic attractions, and are affected by the masses of the entities. Hydrogen is non-polar and has only one proton and one electron per atom, so the electrical attractions among molecules are very weak. For this reason, hydrogen has an extremely low boiling point and so it is difficult to liquefy. 47. (a) Entities slow down as temperature decreases. As the temperature approaches absolute zero, motion also slows until the entities effectively have no motion. (b) If entities have very little motion, they cannot overcome the intermolecular forces among them, and they tend to stick together. 48. If the gases react with each other, the amount of each reactant gas decreases, as does its partial pressure. In addition, the overall number of entities is likely to change, which would change the total pressure of the mixture. 49. The total pressure of the mixture is the sum of the partial pressures: 5.0 kPa + 19 kPa + 79 kPa = 103 kPa. 50. (a) Given: partial pressure of hydrogen gas, PH = 98.5 kPa 2 temperature, t = 23 ºC vapour pressure of water at 23 ºC (Table 1), PH O = 2.81 kPa 2 Required: total pressure, Ptotal Analysis: Use the equation for Dalton’s law of partial pressures. Ptotal = PH + PH O 2 2 Solution: Ptotal = PH + PH O 2 Ptotal 2 = 98.5 kPa + 2.81 kPa = 101.3 kPa Statement: The total pressure in the test tube is 101.3 kPa. (b) The conditions are the same in both test tubes, so the total pressures should be equal. 51. Given: partial pressure of carbon dioxide, PCO = 152 kPa 2 temperature, t = 17.0 ºC vapour pressure of water at 17 ºC (Table 1), PH O = 1.94 kPa 2 Required: total pressure, Ptotal Analysis: Use the equation for Dalton’s law of partial pressures. Ptotal = PCO + PH O 2 2 Copyright © 2011 Nelson Education Ltd. Chapter 12: Gas Laws, Gas Mixtures, and Gas Reactions 12-8 Solution: Ptotal = PCO + PH O 2 2 = 152 kPa + 1.94 kPa = 154 kPa Ptotal Statement: The total pressure of the gases in the bottle is 154 kPa. 52. (a) 2 C4 H10 (g) + 13 O 2 (g) ! 8 CO 2 (g) + 10 H 2O(g) (b) From the balanced equation, the volume ratio of butane to oxygen is 2 : 13. So 4.0 L of butane reacts completely with 26 L of oxygen. (c) The volume ratio of butane to water vapour is 2 : 10, or 1 : 5. So 4.0 L of butane produces 2.0 × 10 L of water vapour. (d) The volume ratio of butane to carbon dioxide is 2 : 8, or 1 : 4. So 4.0 L of butane produces 16 L of carbon dioxide. 53. (a) 2 C2 H 6 (g) + 7 O 2 (g) ! 4 CO 2 (g) + 6 H 2O(g) (b) The mole ratio from the balanced equation is 2 : 7 : 4 : 6. The mole ratio is also the volume ratio if the temperature and pressure remain constant. The ratio of the total volume of reactants to total volume of products is 9 : 10. At constant conditions, the volume of products is slightly greater than the volume of reactants. (c) Given: mass of ethane, mC H = 56.0 g 2 6 molar mass of ethane, M C H = 30.08 g/mol 2 6 The conditions are standard temperature and pressure, so temperature, T = 273 K, and pressure, P = 101.325 kPa. Required: volume of water vapour, VH O 2 Solution: Step 1. Write the balanced chemical equation, listing the given and required quantities. 2 C2 H 6 (g) + 7 O 2 (g) ! 4 CO 2 (g) + 6 H 2O(g) 56.0 g VH O 2 Step 2. Convert the mass of the given substance, mC H , into an amount, nC H . 2 nC H = 56.0 g ! 2 6 6 2 6 1 mol 30.08 g nC H = 1.8617 mol 2 6 [two extra digits carried] Step 3. Determine the amount of water vapour produced from the amount of ethane used. 6 molH O 2 nH O = 1.8617 mol ! 2 2 molC H 2 nH O = 5.5851 mol 2 6 [two extra digits carried] Copyright © 2011 Nelson Education Ltd. Chapter 12: Gas Laws, Gas Mixtures, and Gas Reactions 12-9 Step 4. Determine the volume of the required substance, VH O , using the ideal gas law equation, –1 –1 2 PV = nRT. Remember that R = 8.314 kPa i L i mol i K . PVH O = nH O RT 2 2 VH O = 2 = nH O RT 2 P (5.5815 mol ) !" 8.314 kPa iLi mol –1 ( –1 i K # 273 K $ ) 101.325 kPa VH O = 125 L 2 Statement: When 56.0 g of ethane reacts with excess oxygen at STP, 125 L of water vapour is produced. (d) Given: mass of oxygen, mO = 78 g 2 molar mass of oxygen, M O = 32.00 g/mol 2 temperature, t = 24.0 ºC pressure, P = 98 kPa Required: volume of carbon dioxide, VCO 2 Solution: Step 1. Convert the temperature value to kelvins. T = t + 273 = 24.0 + 273 T = 297.0 K Step 2. Write the balanced chemical equation, listing the given and required quantities. 2 C2 H 6 (g) + 7 O 2 (g) ! 4 CO 2 (g) + 6 H 2O(g) 78 g VCO 2 Step 3. Convert the mass of the given substance, mO , into an amount, nO . 2 nO = 78 g ! 2 2 1 mol 32.00 g nO = 2.4374 mol 2 [two extra digits carried] Step 4. Determine the amount of carbon dioxide produced from the amount of oxygen used. 4 molCO 2 nCO = 2.4374 mol ! 2 7 molO 2 nCO = 1.3929 mol 2 [two extra digits carried] Copyright © 2011 Nelson Education Ltd. Chapter 12: Gas Laws, Gas Mixtures, and Gas Reactions 12-10 Step 5. Determine the volume of the required substance, VCO , using the ideal gas law equation, –1 –1 2 PV = nRT. Remember that R = 8.314 kPa i L i mol i K . PVCO = nCO RT 2 2 VCO = 2 = nCO RT 2 P (1.3929 mol ) !" 8.314 kPa iLi mol –1 ( i K –1 # 297.0 K $ ) 98 kPa VCO = 35 L 2 Statement: When 78 g of oxygen reacts with ethane, 35 L of carbon dioxide is produced (under the given conditions of temperature and pressure). Analysis and Application 54. (a) Equal volumes of gases under the same conditions contain equal numbers of entities. The two balloons therefore contain the same number of entities. (b) Since the temperature is the same in both balloons, the average kinetic energy of the entities must be the same. Thus, the pressure is the result of only the number of entities present. The pressure is the same, so the number of entities is the same. 55. Given: volume, V = 10.0 m × 7.0 m × 3.0 m The conditions are standard ambient temperature and pressure Required: amount of radon, n Analysis: The conditions are SATP, so the conversion factor derived from the molar volume at SATP may be used. In this case we wish to find the amount of radon, n, so we use the factor 1 mol/24.8 L as follows: 1 mol n=V ! 24.8 L Solution: Step 1. Determine the volume of the gas, in litres. Recall that 1000 L = 1 m3. 1000 L V = 10.0 m ! 7.0 m ! 3.0 m ! 1 m3 1000 L = 210 m 3 ! 1 m3 V = 210 000 L Step 2. Substitute in the known values and solve the mathematical equation. 1 mol n = 210 000 L ! 24.8 L 3 n = 8.5 ! 10 mol Statement: The amount of radon gas in the basement is 8.5 × 103 mol. Copyright © 2011 Nelson Education Ltd. Chapter 12: Gas Laws, Gas Mixtures, and Gas Reactions 12-11 56. 57. Given: initial volume, V1 = 20.4 L initial temperature, t1 = 25.0 ºC initial pressure, P1 = 101 kPa final temperature, t2 = 28.0 ºC final pressure, P2 = 98.0 kPa R = 8.314 kPa i L i mol–1 i K–1 Required: final volume, V2 Analysis: First use the ideal gas law equation to calculate the amount of gas in the balloon. P1V1 = nRT1 Then use this amount of gas to calculate the final volume of the balloon. P2V2 = nRT2 Solution: Step 1. Convert the temperature values to kelvins. T1 = t1 + 273 = 25.0 + 273 T1 = 298.0 K T2 = t2 + 273 = 28.0 + 273 T2 = 301.0 K Step 2. Rearrange the ideal gas law equation to find the amount of gas. PV n= 1 1 RT1 Step 3. Substitute known values into the equation and solve. n= 101 kPa ! 20.4 L 8.314 kPa i L imol–1 i K –1 ! 298.0 K n = 0.83162 mol [two extra digits carried] Copyright © 2011 Nelson Education Ltd. Chapter 12: Gas Laws, Gas Mixtures, and Gas Reactions 12-12 Step 4. Rearrange the ideal gas law equation to find the final volume. nRT2 V2 = P2 Step 5. Substitute known values into the equation and solve. V2 = (0.83162 mol)(8.314 )( kPa i L imol–1 i K –1 301.0 K ) 98.0 kPa V2 = 21.2 L Statement: The final volume of the balloon was 21.2 L. 58. Given: total volume of container, Vtotal = 250 mL volume of liquid bromine, Vliquid = 25.0 mL t = 20.0 ºC P = 102 kPa R = 8.314 kPa i L i mol–1 i K–1 M Br = 159.80 g/mol 2 Required: mass of bromine, mBr 2 Analysis: First find the volume of bromine vapour in the container. Then use the ideal gas law equation to calculate the amount of bromine vapour in the container. PV = nRT Then convert the amount to mass. Solution: Step 1. Determine the amount of bromine vapour in the container. Vtotal = Vliquid + Vvapour Vvapour = Vtotal ! Vliquid Vvapour = 250 mL ! 25.0 mL = 225.0 mL [two extra digits carried] Step 2. Convert the temperature value to kelvins and volume from mL to L. T = t + 273 = 20.0 + 273 T = 293.0 K Vvapour = 225.0 mL = 225.0 mL ! Vvapour = 0.2250 L 1L 1000 mL [two extra digits carried] Step 3. Rearrange the ideal gas law equation to find the amount of vapour. PV n= RT Copyright © 2011 Nelson Education Ltd. Chapter 12: Gas Laws, Gas Mixtures, and Gas Reactions 12-13 Step 4. Substitute known values into the equation and solve. 102 kPa ! 0.2250 L n= –1 8.314 kPa i L imol–1 i K ! 293.0 K n = 0.009 421 mol [two extra digits carried] Step 5. Convert amount into mass using the appropriate conversion factor involving molar mass. 159.80 g mBr = nBr ! 2 2 1 mol 159.80 g = 0.009 421 mol ! 1 mol mBr = 1.5 g 2 Statement: The container contains 1.5 g of bromine vapour. 59. (a) The line representing the behaviour of a real gas at 100 K would initially dip deeper than the line for a real gas at 200 K, and the part of the line going up would be steeper than the line for 200 K. The line for a real gas at 100 K would probably reach a value of 3 on the y-axis before it reaches a value of 600 atm on the x-axis. (b) A real gas most closely approaches the behaviour of an ideal gas at high temperatures and low pressures. 60. (a) Given: V = 500.0 mL R = 8.314 kPa i L i mol–1 i K–1 m = 1.55 g t = 25.0 ºC P = 240 kPa Required: molar mass of gas, M Analysis: First, use the ideal gas law to find the amount of gas. PV = nRT Next, use the amount of gas to determine the molar mass. Solution: Step 1. Convert the temperature value to kelvins and volume from mL to L. T = t + 273 = 25.0 + 273 T = 298.0 K V = 500.0 mL = 500.0 mL ! 1L 1000 mL V = 0.5000 L Step 2. Rearrange the ideal gas law equation to isolate the unknown variable, n. PV n= RT Copyright © 2011 Nelson Education Ltd. Chapter 12: Gas Laws, Gas Mixtures, and Gas Reactions 12-14 Step 3. Substitute known values into the equation and solve. n= 240 kPa ! 0.5000 L 8.314 kPa i L imol–1 i K –1 ! 298.0 K n = 0.048 43 mol [two extra digits carried] Step 4. Find the molar mass of the gas by rearranging the values for mass and amount. 1.55 g M= 0.048 43 mol M = 32 g/mol Statement: The molar mass of the gas is 32 g/mol. (b) Since the molar mass of O2 is 32.00 g/mol, the gas must be oxygen. 61. The total pressure of gas in the tank is the sum of the partial pressure of nitrogen gas, the partial pressure of oxygen gas, and the partial pressure of any other gases present. The partial pressures will vary depending on the proportion of the various gases and on the overall pressure in the tank. 62. (a) The molar ratio of carbon dioxide and oxygen is 1 : 1, so the volume ratio must also be 1 : 1 since the pressure and temperature remain constant. So 12.7 L of carbon dioxide produces 12.7 L of oxygen. (b) The student’s statement is not correct. Coefficients in the equation are ratios of amounts or volumes, not masses. The masses are not in a 1 : 1 ratio because the molar mass differs for each substance. The mass of carbon dioxide consumed will be greater than the mass of oxygen produced. 63. (a) C6 H12O6 (aq) ! 2 CH 3CH 2OH(l) + 2 CO 2 (g) + energy (b) Given: amount of glucose, nC H 6 12 O6 = 2.00 mol The conditions are standard temperature and pressure, so T = 273 K and P = 101.325 kPa. Required: volume of carbon dioxide, VCO 2 Solution: Step 1. Write the balanced chemical equation, listing the given and required quantities. C6 H12O6 (aq) ! 2 CH 3CH 2OH(l) + 2 CO 2 (g) + energy 2.00 mol VCO 2 Step 2. Determine the amount of carbon dioxide produced from the amount of glucose used. 2 molCO 2 nCO = 2.00 mol ! 2 1 molC H O 6 12 6 nCO = 4.00 mol 2 Copyright © 2011 Nelson Education Ltd. Chapter 12: Gas Laws, Gas Mixtures, and Gas Reactions 12-15 Step 3. Determine the volume of the required substance, VCO , using the ideal gas law equation, –1 2 –1 PV = nRT. Remember that R = 8.314 kPa i L i mol i K . PVCO = nCO RT 2 2 VCO = 2 = nCO RT 2 P (4.00 mol ) !" 8.314 kPa iLi mol –1 ( i K –1 # 273 K $ ) 101.325 kPa VCO = 89.6 L 2 Statement: When yeast and bacteria process 2.00 mol of glucose at STP, 89.6 L of carbon dioxide is produced. (c) Given: mass of ethanol, mCH CH OH = 82 g 3 2 molar mass of ethanol, M CH CH OH = 46.08 g 3 2 The conditions are standard temperature and pressure, so T = 273 K and P = 101.325 kPa. Required: mass of carbon dioxide, mCO 2 Solution: Step 1. Write the balanced chemical equation, listing the given and required quantities. C6 H12O6 (aq) ! 2 CH 3CH 2OH(l) + 2 CO 2 (g) + energy 82 g mCO 2 Step 2. Determine the amount of the given substance, nCO . 2 1 mol 3 2 46.08 g 1 mol = 82 g ! 46.08 g nCH CH OH = mCH CH OH ! 3 2 nCH CH OH = 1.780 mol 3 2 [two extra digits carried] Step 3. Determine the amount of carbon dioxide that is produced with the amount of ethanol. 2 molCO 2 nCO = 1.780 mol ! 2 2 molCH CH OH 3 2 nCO = 1.780 mol 2 Copyright © 2011 Nelson Education Ltd. Chapter 12: Gas Laws, Gas Mixtures, and Gas Reactions 12-16 Step 4. Determine the mass of carbon dioxide that is produced from the amount of carbon dioxide. M CO = 44.01 g/mol 2 44.01 g 1 mol 44.01 g = 1.780 mol ! 1 mol = 78 g mCO = nCO ! 2 mCO 2 2 Statement: 78 g of carbon dioxide is produced when 82 g of ethanol is produced at STP. (d) Large volumes of gases are produced during fermentation. The pressure exerted by those gases in a closed container could cause the container to burst. Evaluation 64. (a) The estimate does not make sense. At the same temperature and pressure, a smaller volume of gas would contain a smaller amount of gas, not a larger amount. Vn (b) The meterologist used Avogadro’s law incorrectly. She used the equation n2 = 1 1 instead V2 V2 n1 . V1 (c) The correct amount of gas that should be added to the second balloon can be found from the correct use of Avogadro’s law. V1 V2 = n1 n2 of the correct equation n2 = n2 = = V2 n1 V1 2.60 kL ! 140.0 mol 3.10 kL n2 = 117 mol The amount of gas that should be added to the second balloon is 117 mol. 65. The student is not correct because not all three compounds involved in the reaction are gases. The volume relationships that apply to gases do not apply to other states of matter. 66. The numerical value used for R has a unit based on units of kPa for pressure. The student used atm for pressure instead of kPa. Replacing 1.00 atm with 101.3 kPa will give the correct answer. 67. (a) PAr = 20.0 % ! Ptotal = 0.200 ! 158 kPa PAr = 31.6 kPa The partial pressure of argon is 31.6 kPa. Copyright © 2011 Nelson Education Ltd. Chapter 12: Gas Laws, Gas Mixtures, and Gas Reactions 12-17 PHe = 80.0 % ! Ptotal = 0.800 ! 158 kPa PHe = 126 kPa The partial pressure of helium is 126 kPa. (b) From Boyle’s law, P1V1 = P2V2, where P1 and V1 are the pressure and volume, respectively, in the initial container and P2 and V2 are the pressure and volume, respectively, in the new P container. In this situation V2 = 2V1, so P1V1 = P2(2V1) which gives us P2 = 1 . The pressure in 2 the new container, P2, can now be found. P P2 = 1 2 158 kPa = 2 P2 = 79.0 kPa The percentages of the gases do not change, so the partial pressures can now be found similarly to part (a) using the new total pressure. (c) PAr = 20.0 % ! Ptotal = 0.200 ! 79.0 kPa PAr = 15.8 kPa The partial pressure of argon is 15.8 kPa. PHe = 80.0 % ! Ptotal = 0.800 ! 79 kPa PHe = 63.2 kPa The partial pressure of helium is 63.2 kPa. 68. (a) The chemical equation for cellular respiration is the reverse of the chemical equation for photosynthesis. (b) If more energy is released during and after exercising, more oxygen must be inhaled, and more carbon dioxide is exhaled during and immediately after exercising. Reflect on Your Learning 69. According to the ideal gas law, PV = nRT, if pressure and temperature remain the same, a difference in volume must result from a difference in the number of entities. Some gas must have leaked out of the balloons that became smaller. 70. Ideal gases do not liquefy because there are no intermolecular attractions in an ideal gas. Real gases do liquefy when intermolecular attractions are strong enough (usually at high pressures, which is the case with an oxygen tank). Copyright © 2011 Nelson Education Ltd. Chapter 12: Gas Laws, Gas Mixtures, and Gas Reactions 12-18 71. Answers may vary. Sample answer: Dewar was reluctant to share information with others working in the same field. If he had worked cooperatively, it is possible that the researchers could have achieved their goal earlier. The explosion that injured Dewar’s assistants was the result of unsafe equipment. Dewar’s criticism of other scientists caused them to deny him access to helium, slowing down his research. 72. (a) As water forms from the gases, the concentration of the gases decreases. (b) Answers may vary. Sample answer: An electric current could be passed through the water formed, producing more oxygen and hydrogen gases. 73. (a) Answers may vary. Sample answer: Oxygen, which is produced during photosynthesis, is essential for life. Without oxygen, most organisms on Earth would not survive. Also, carbon dioxide is necessary for photosynthesis, which provides oxygen. Carbon dioxide is also a greenhouse gas that helps to keep Earth warm. However, too much carbon dioxide is causing global warming, which has a significant effect on the environment in many parts of the world. (b) As rainforests are cleared, less carbon dioxide is removed from the atmosphere, and less oxygen is produced. The chemical equation does not change, but the reaction occurs less frequently as the rainforests are destroyed, resulting in an excess of carbon dioxide in the atmosphere. Research 74. (a) Methane is the main component of natural gas. (b) Water and carbon dioxide are produced. A balanced chemical equation for this reaction is CH 4 (g) + 2 O 2 (g) ! CO 2 (g) + 2 H 2O(g) . (c) From the balanced chemical equation, the mole ratio of methane to oxygen is 1 : 2, so if 2 mol of methane are burned, 4 mol of oxygen are used. The mole ratio of methane to carbon dioxide is 1 : 1 and the mole ratio of methane to water vapour is 1 : 2, so if 2 mol of methane are burned, 2 mol of carbon dioxide and 4 mol of water vapour are produced. 75. (a) A fermionic condensate is a state of matter of a type of particle (fermion) that is condensed at extremely cold temperatures. (b) Both condensates are states of matter that exist at temperatures close to absolute zero. A Bose-Einstein condensate involves condensation of particles called bosons. A photon is an example of a boson. Bosons are more easily condensed than fermions because any number of bosons can occupy an energy level. Fermionic condensates involve condensation of particles called fermions. Electrons, protons, and neutrons are examples of fermions. Fermions are more difficult to condense because the number of these particles per energy level is limited. (c) Scientists predict practical uses for condensates in the fields of superconductivity and superfluidity. 76. (a) Most anesthetics used are halogenated ethers. The ethers isoflurane, desflurane, and sevoflurane are most commonly used. (b) The carrier gas used is dinitrogen monoxide (nitrous oxide). (c) Answers may vary. Sample answer: The anesthetic agent must be diluted by the carrier gas so that only a small amount of anesthetic agent is administered at a time. (d) The volatile halogenated ethers used as anesthetics are greenhouse gases, as is the most common carrier of these ethers, dinitrogen monoxide. Only a small amount of the anesthetic gas used is metabolized by the patient. The remainder is released into the atmosphere. Copyright © 2011 Nelson Education Ltd. Chapter 12: Gas Laws, Gas Mixtures, and Gas Reactions 12-19 77. Answers may vary. Students’ answers should include the following points: • Carbon dioxide is a greenhouse gas that contributes to global warming. Making large quantities of carbon dioxide hydrate would remove large amounts of this greenhouse gas from the atmosphere. • Depleted oil sand gas reservoirs in northern Alberta are being considered as storage sites. • Carbon dioxide gas can be pumped into underground supplies of methane hydrate. Due to the low temperatures, carbon dioxide hydrate starts to form. The formation of this hydrate releases enough energy to melt the methane hydrate already there, releasing the methane it contains. Copyright © 2011 Nelson Education Ltd. Chapter 12: Gas Laws, Gas Mixtures, and Gas Reactions 12-20