5.9 Polyatomic Ions
advertisement

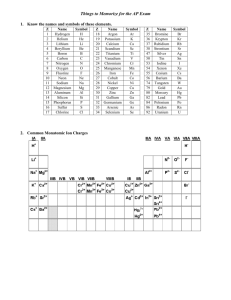
5.9 Polyatomic Ions sodium nitrite preservative and colour enhancer sodium phosphate binding agent sodium erythrobate preservative Figure 1 Hot dogs contain many chemical additives. 3– O O P O O Figure 2 The phosphate ion is made up of four oxygen atoms bonded to a central phosphorus atom. polyatomic ion an ion made up of more than one atom that acts as a single particle C05-F29-UBOS10SB.ai DID YOU KNOW? Not Just for Baking Sodium hydrogen carbonate (baking soda) is an extremely versatile substance. It can substitute for toothpaste if you run out, it soaks up odours from your shoes and refrigerator, it is a good abrasive cleaner, and it is even useful to help relieve insect bites and sunburn. GO TO NELSON SCIENCE 202 Processed foods contain a lot of sodium, mostly from sodium chloride, NaCl (table salt). Sodium chloride enhances the flavour and extends the shelf-life of food. Other additives in processed foods also contribute to your daily sodium intake (Figure 1). For example, sodium phosphate helps bind together the meat in a hot dog. Sodium nitrite acts as a preservative and flavour enhancer and gives hot dogs their pinkish colour. There is some concern about adding compounds containing nitrite ions to foods. Nitrite ions react with substances in the digestive tract to form nitrosamines in the body. Nitrosamines are chemicals that have been linked to certain types of cancer in laboratory animals. An occasional hot dog is unlikely to make you ill, but a steady diet of processed meats is not recommended. Nitrites are not always bad news, however. Research suggests that sodium nitrite may help protect heart tissue after a heart attack or transplant. Also, nitrites and a related group of compounds called nitrates are important plant nutrients. They occur naturally in soil and are manufactured and spread as fertilizer on many crops. You can see how difficult it is to make a simple judgment about whether a chemical is good or bad. The sodium compounds in Figure 1 are ionic compounds similar to the others you have learned about in this chapter. Sodium phosphate, for example, is a white solid, relatively stable, and an electrolyte. The chemical formula for this compound is Na3PO4. Its cation is sodium, but its anion, phosphate, (PO4)3–, is an example of a polyatomic ion (Figure 2). A polyatomic ion is an ion that consists of a stable group of several atoms acting together as a single charged particle. The ionic charge of a polyatomic ion is shared over the entire ion rather than being on just one atom. Table 1 lists some of the most common polyatomic ions and their ionic charges. Note that all the ions are anions except ammonium. Also, note that all the anion names end in “ate” except nitrite and hydroxide. Table 1 Formulas and Charges of Common Polyatomic Ions Name of polyatomic ion Ion formula Ionic charge nitrate ion NO3– −1 nitrite ion NO2– −1 hydroxide ion OH– −1 hydrogen carbonate ion (also called bicarbonate ion) HCO3– −1 chlorate ion ClO3– −1 carbonate ion CO32– −2 sulfate ion SO42– −2 phosphate ion PO43– −3 ammonium NH4+ +1 Chapter 5 • Chemicals and Their Properties NEL Naming Compounds Involving Polyatomic Ions The strategy for naming polyatomic compounds uses the same steps that you learned in the previous section. The only difference is that the anion is named according to the polyatomic ion rather than the names of the individual elements. SAMPLE PROBLEM 1 Naming Compounds Involving Polyatomic Ions Write the name of the compound Na2CO3. (Sodium does not form multiple ions.) Step 1 Write the name of the metal and check whether it has more than one ionic charge. Sodium always has a charge of +1. Step 2 Write the name of the compound. The name of Na2CO3 is sodium carbonate. Practice What is the name of Ca(OH)2? As the Practice example indicates, several polyatomic ions can be associated with each cation. This is indicated by placing brackets around the polyatomic ion and writing the subscript outside the brackets. Some metal ions have more than one charge. Check this before naming the compound. In compounds containing these ions, use a Roman numeral to indicate the charge. SAMPLE PROBLEM 2 Naming Compounds Involving Polyatomic Ions Write the name of the compound Fe(NO3)3. Step 1 Write the name of the metal and check whether it has more than one possible ionic charge. If it does, proceed to step 2. If not, proceed to step 3. Iron has two possible ionic charges: +2 and +3. Step 2 Determine the ionic charge of the metal. Fe (NO3)3 x + 3 (−1) = 0 x = +3 The ionic charge of iron in this compound is +3, indicated by the Roman numeral “III.” Step 3 Write the name of the compound, with the Roman numeral if necessary. The name of Fe(NO3)3 is iron(III) nitrate. Practice What is the name of CuSO4? NEL 5.9 Polyatomic Ions 203 Writing Formulas Involving Polyatomic Ions You can apply the rules that you have already learned for writing ionic compound formulas to polyatomic compounds. Remember to treat the polyatomic ion as one unit. Consider the following Sample Problem. SAMPLE PROBLEM 3 Writing Formulas for Compounds Containing Polyatomic Ions What is the chemical formula of sodium phosphate? DID YOU KNOW? Step 1 Write the symbols of the each ion, beginning with the cation (metal). The Phosphate Fiasco In the 1960s, laundry detergents included phosphates. The phosphateladen wastewater was flushed into lakes and streams. Phosphates are an important plant nutrient. The increased dose of nutrients caused unnaturally rapid growth in aquatic plant populations. As these plants died and decomposed, much of the oxygen in the water was used up, killing many aquatic animals. To avoid the problem, chemical engineers redesigned detergents to work effectively without phosphates. Na+ 3– O Na+ O P O O Na+ Figure 3 Sodium phosphate, Na3PO4, consists of three sodium ions and one phosphate ion, PO43−. When this compound dissolves, these four ions separate. However, the phosphate ion remains intact. C05-F30-UBOS10SB.ai Na PO4 Step 2 Write the ionic charges above each ion. +1 Na −3 PO4 Step 3 Determine how many ions of each type are required to give a total charge of zero. Total ionic charge: 3(+1) Na + 1(−3) = 0 PO4 Three sodium ions balance the −3 charge of the phosphate ion. Step 4 Write the chemical formula using the coefficients as subscripts. Na3PO4 The formula of sodium phosphate is Na3PO4. Note that sodium phosphate contains three sodium ions and one phosphate ion (Figure 3). The “crisscross method” also works for compounds with polyatomic ions. Sample Problem 4 shows how. Note that, if a coefficient is required for the polyatomic ion, first put curved brackets around it and then write the subscript outside of the final bracket. SAMPLE PROBLEM 4 Writing Formulas for Compounds Containing Polyatomic Ions What is the chemical formula of copper(II) nitrate? Step 1 Write the symbol of each ion, with its charge. Cu2+ (NO3)– Step 2 Crisscross the numbers of the ionic charges so that they now become subscripts. (NO3)– Cu2+ Step 3 Write any necessary subscripts after the brackets around each ion. (Remember, you do not need to write “1” as a subscript, so no brackets are necessary.) Cu (NO3)2 The formula of copper(II) nitrate is Cu(NO3)2. Practice What is the chemical formula of ammonium carbonate? 204 Chapter 5 • Chemicals and Their Properties NEL IN SUMMARY • Polyatomic ions are made up of more than one atom, with a charge spread over the entire ion. • Polyatomic ions are present in many natural and artificial compounds. They are used as food additives, fertilizers, and cleaners. CHECK YOUR LEARNING 1. Name the polyatomic ion in each of the following compounds and name the compound. (Watch for metals with more than one possible ionic charge.) K/U (a) KNO3 (found in gun powder) (b) Ca(OH)2 (an ingredient in plaster) (c) CaCO3 (in chalk, limestone, and antacid medicines) 6. Write the chemical formulas of the following compounds. Note that some of them contain polyatomic ions. K/U (a) calcium sulfate (e) calcium chlorate (b) ammonium chloride (f) tin(II) hydroxide (c) copper(I) carbonate (g) iron(IV) phosphate (d) barium sulfide (h) aluminum nitride 7. Explain why the chemical formula for calcium hydroxide, Ca(OH)2, is not written as CaO2H2. K/U (d) CuSO4 (a fungicide) (e) KOH (used to make soap) 8. Most ionic compounds are made up of a metal cation and a non-metal anion. Look at Table 1 on page 202 and identify an exception to this rule. K/U (f) Fe(NO3)3 (used in water treatment) (g) Cu(ClO3)2 (used to colour fireworks) (h) (NH4)3PO4 (an ingredient in bread dough) 2. Write the chemical formula for each of the following compounds: K/U (a) potassium nitrate (used to colour fireworks purple) (b) barium sulfate (given prior to an X-ray of the intestine) (c) ammonium nitrate (a common ingredient in fertilizer) 9. When writing the chemical formula of an ionic compound, which ion is always written first? K/U 10. Copy Table 2 into your notebook. Complete your table using the example provided as a guide. K/U Table 2 Identifying Ions (d) aluminum sulfate (used in preparing pickles) Compound (e) potassium chlorate (an explosive) Fe(OH)3 (f) copper(II) nitrate (used to colour ceramics) Cu(NO3)2 (g) lead(II) sulfate (found in car batteries) Al2(SO4)3 (h) tin(II) phosphate (used in the dyeing of silk) (NH4)2CO3 3. What is the most common ending for the name of (a) a polyatomic anion? (b) an anion made up of only one element? K/U 4. Well water in agricultural areas is often monitored for nitrate ions because of possible health effects. Where can nitrate contamination come from on a farm? A 5. Write the names of the following compounds. Note that some of them contain polyatomic ions. K/U (a) SnCO3 (e) K2S (b) CaCl2 (f) (NH4)2SO4 (c) Fe(OH)3 (g) Mn(ClO3)2 (d) MnO2 NEL • Consider the polyatomic ion as a single unit when writing the chemical formula for a compound that includes a polyatomic ion. If the compound contains more than one of the polyatomic ion, write curved brackets around the ion and write a subscript outside the brackets. Cation(s) Anion(s) 1 Fe3+ 3 OH– K3PO4 11. The names “sodium chloride” and “sodium chlorate” sound similar. However, these compounds are very different. Sodium chloride enhances the flavour of food while sodium chlorate is a toxic herbicide. For each compound, write (a) the chemical formula (b) the chemical formula of the anion (c) the chemical formula of a compound that this anion makes with calcium K/U 12. Describe one strategy you can use to reduce the amount of salt in your diet. A (h) PbI2 5.9 Polyatomic Ions 205