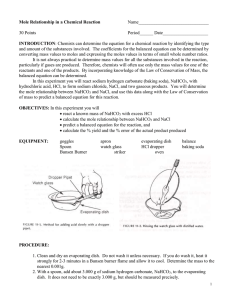
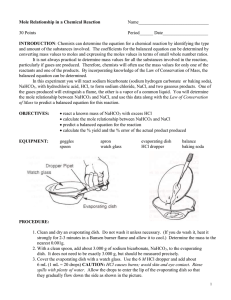
Name _________________________________ Stoichiometry: The Relationship between Moles and Mass PURPOSE: To compare the ACTUAL (experimental) mass to the THEORETICAL mass of a product of a chemical reaction. INTRODUCTION: Given the mole relationships in a balanced chemical equation, and the mass of one of the reactants, it is possible to determine the number of moles of every other substance taking part in the reaction. In this experiment, you will investigate the following chemical reaction: NaHCO3(s) + HCl(aq) NaCl(aq) + CO2(g) + H2O(l) A known mass of sodium hydrogen carbonate (baking soda) will be reacted with excess hydrochloric acid. Knowing the mass of sodium hydrogen carbonate that reacts, you can determine from the balanced equation the mass of NaCl that should be produced. You can compare this theoretical value with the actual experimental mass of NaCl produced. MATERIALS AND EQUIPMENT: Sodium bicarbonate 6M hydrochloric acid hot plate microspatula balance evaporating dish SAFETY PRECAUTIONS: 1. Safety goggles and lab aprons are required for this lab. 2. Long hair should be tied back and loose clothing secured when working with a Bunsen burner. 3. Do not touch a hot evaporating dish with your hands. 4. Handle hydrochloric acid with care. Flush any spills with cold water and report to the teacher. 5. Do not lean over the apparatus when heating it in step 6. PROCEDURE: 1. Find the mass of the evaporating dish. Record this mass in your data table. 2. Measure out 2.50 grams of sodium hydrogen carbonate (NaHCO3). Place this in the evaporating dish. Record the combined mass of the sodium hydrogen carbonate and the dish in the data table below. 3. Place the evaporating dish (containing sodium hydrogen carbonate) on the counter top. Do NOT pick up the 6M HCl by the dropper. Pick it up by the glass bottle and place it next to the evaporating dish 4. Using a dropper, slowly add 6 M HCl to the sodium hydrogen carbonate in the evaporating dish, a few drops at a time, until the sodium bicarbonate is totally dissolved. There will be bubbles, allow the bubbles to stop before adding more HCl. Do NOT use the dropper to stir the mixture. 5. Once the solid is dissolved, continue adding acid, drop by drop, until the reaction is complete (bubbling stops.) Carefully tilt the evaporating dish back and forth a couple of times to make sure that the acid has contacted all the sodium hydrogen carbonate. Do NOT use more HCl than is needed, this will give you a high percent yield. 6. Secure the dropper in the bottle and place the bottle back into the lab bin 7. Place the evaporating dish on the hot plate and turn the hot plate on to 250. Heat gently until no liquid remains. Gently poke a few holes in the solid and break up the clumps to allow moisture to escape and continue to heat until it looks like a powder, 8. Once the solid looks like a white powder, turn the hot plate up to 400. Use the tip of the tongs to secure the evaporating dish and continue to break up and move the solid around to release the rest of the moisture. (about 3-4 more minutes) You should be able to hear the water leaving at first. After a few minutes the sound should decrease. Allow the dish to cool. 8. While this process is going on you can start answering most of the lab questions that do not require your actual yield. 9. Find the combined mass of the evaporating dish and contents (NaCl). Record this mass in the data table. 10. CLEAN UP: use a damp paper towel to wipe out the NaCl from the evaporation dish into the trash can. Then use one of the big sinks to rinse out the evaporating dish, then use a dry paper towel to completely dry the dish. Place the dish and all other lab equipment back in the lab bin. DATA AND CALCULATIONS: Object Mass of empty evaporating dish Mass of dish + NaHCO3 (sodium hydrogen carbonate) Mass of NaHCO3 reactant Mass of dish + NaCl (after reaction and heating) Mass of NaCl product Mass (grams) CONCLUSIONS: 1. According to the balanced equation used in this experiment, what is the ratio of moles of NaHCO3 reacted to moles of the NaCl produced? NaHCO3(s) + HCl(aq) NaCl(aq) + CO2(g) + H2O(l) 2. How many moles of NaHCO3 is reacted in this experiment? Look in your data table and convert the grams of NaHCO3 to moles. 3. How many moles of NaCl are produced? Look in your data table and convert the grams of NaCl to moles. 4. What is the ratio of moles of NaHCO3 reacted to moles of NaCl produced according to your lab results? 5. Calculate the mass of NaCl you would expect to get when 2.50 g of NaHCO3 is reacted with HCl. 6. Calculate your percent yield for the mass of NaCl. What does this mean? 7. Calculate your percent error. 8. Calcium carbonate decomposes to form calcium oxide and carbon dioxide. If 40 g of CaCO3 is decomposed: CaCO3 CaO + CO2 a. How many grams of CaO will be produced? b. How many liters of CO2 will be produced at STP? 9. Nitrogen and hydrogen combine to form ammonia (NH3): N2 + 3H2 2NH3 If 20 g of hydrogen react: a. How many grams of NH3 are produced? b. How many grams of N2 react? Stoichiometry: The Relationship between Moles and Mass Answers DATA TABLE: _______________ Mass of dish _______________ Mass of dish and sodium hydrogen carbonate _______________ Mass of dish and NaCl _______________ Mass of the sodium hydrogen carbonate reactant = 2.50 g _______________ Mass of the NaCl product = 1.62 g CONCLUSIONS: 1. According to the balanced equation for the reaction (found in the introduction) used in this experiment, what is the ratio of moles of sodium hydrogen carbonate reacted to moles of the NaCl produced? It is a 1:1 ratio. For every mole of sodium hydrogen carbonate reacted, there will be one mole of NaCl produced. 2. How many moles of sodium hydrogen carbonate is reacted in this experiment? Look in your data table and convert the grams of sodium hydrogen carbonate to moles. 2.5 g NaHCO3 1 mol NaHCO3 = .030 mol NaHCO3 84.01 g NaHCO3 3. How many moles of NaCl are produced? Look in your data table and convert the grams of NaCl to moles. 1.62 g NaCl 1 mol NaCl = .0277 mol NaCl 58.44 g NaCl 4. What is the ratio of moles of sodium hydrogen carbonate reacted to moles of NaCl produced according to your lab results? The ratio is very close to the expected 1:1 ratio. 5. Calculate the mass of NaCl you would expect to get when 2.5 g of sodium hydrogen carbonate is reacted with HCl. How does this value compare with the mass attained experimentally? 2.5 g NaHCO3 1 mol NaHCO3 1 mol NaCl 58.44 g NaCl 84.01 g NaHCO3 1 mol NaHCO3 1 mol NaCl = 1.74 g NaCl 6. Calculate your percent error. % Error = True Value - Experimental Value x 100% True Value % Error = 1.74 g NaCl - 1.62 g NaCl x 100% = 6.90 % Error 1.74 g NaCl 7. Calcium carbonate decomposes to form calcium oxide and carbon dioxide. If 40 g of calcium carbonate is decomposed, how many grams of calcium oxide will be produced? CaCO3 40 g CaCO3 CaO + CO2 1 mol CaCO3 1 mol CaO 56.08 g CaO 100.09 g CaCO3 1 mol CaCO3 1 mol CaO = 22.41 g CaO 8. Nitrogen and hydrogen combine to form ammonia (NH3). If 20g of hydrogen react, how many grams of ammonia are produced? How many grams of nitrogen react? N2 + 3H2 2 NH3 20 g H2 1 mol H2 2 mol NH3 17.04 g NH3 = 112.48 g NH3 20 g H2 2.02 g H2 3 mol H2 1 mol NH3 1 mol H2 1 mol N2 28.02 g N2 2.02 g H2 3 mol H2 1 mol N2 = 92.48 g N2