Galvanic Cells 1/4/2010
advertisement

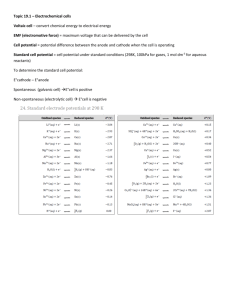
1/4/2010 Electrochemical Cells Galvanic Cells Voltaic / Galvanic Cell Apparatus which produce electricity Electrolytic Cell Apparatus which consumes electricity Consider: Zn Initially there is a flow of eAfter some time the process stops Electron transport stops because of charge build up Cu Chapter 9.5 Build up of positive charge Build up of negative charge The charge separation will lead to process where it cost too much energy to transfer electron. Sign Convention of Voltaic Cell Completing the Circuit Electron transfer can occur if the circuit is closed Parts: @ Anode: Negative Terminal (anions). Source of electron then repels electrons. Oxidation occurs. Zn(s) g Zn+2(aq) + 2e- : Electron source @ Cathode: Positive Terminal (cation) Attracts electron and then consumes electron. Reduction occurs. Electron target: 2e- + Cu +2(aq) g Cu(s) 3 process must happen if e- is to flow. Two conductors A. e- transport through external circuit B. In the cell, ions must migrate Electrolyte solution Salt Bridge / Porous membraneC. Circuit must be closed (no charge build up) Anode (-) Cathode (+) A Black Overall: Red Negative electrode generates electron Zn(s) + Cu+2(aq) g Zn+2(aq) + Cu(s) Positive electrode accepts electron C B Oxidation Occur Sign of E is also reversed E = -1.10 V Zn(s) g Zn +2(aq) Oxidation: Reduction: Cu +2(aq) g Reduction Occur Cathode/Cation(+) CELL = E red (Cathode) - E Line notation: Convenient convention for electrochemical cell Consider : Schematic Representation Anode: Zn g Zn+2 + 2e- Cathode: 2. Anode [oxidation (-)] E = 0.34 V ox CELL (anode) Line Notation Examples Line Notation Convention 1. E = 0.76 V Cu(s) 1.10 V = E or E Anode/Anion (-) E = 1.10 V Note when the reaction is reverse: Cu(s) + Zn+2(aq) g Cu+2(aq) + Zn(s) Cathode [reduction (+)] “ “ phase boundary Zn(s) + Cu +2(aq) g Zn+2(aq) + Cu (s Cu+2 + 2e- g Cu Shorthand “Line” notation Zn (s) Zn+2 (aq)(1.0M) Cu+2(aq) (1.0M) Cu(s) (where potential may develop) 2nd Example : Zn(s) + 2H+ (aq) g Zn+2(aq) + H2(g) 3. “ “ Liquid junction 4. Concentration of component Anode: Zn g Zn+2 + 2e- Cathode: 2H+ + 2e- g H2 (g) Shorthand “Line” notation 4 1 Zn(s) ZnSO4 (aq,1.0M) 3 CuSO4 (aq,1.0M) Cu(s) Zn (s) Zn+2 (aq)(1.0M) H+(aq) (1.0M), H2(g, 1atm) Pt(s) 2 1 1/4/2010 Other Voltaic Cell & Their Line Notation Zn(s) | Zn+2 (aq)||H+(aq) , H2 (g,1atm)|Pt C(s)| I-(aq) , I2 (g,1atm) || MnO4-(aq) , Mn+2 Cr(s) | Cr+3 (aq)||Ag+(aq) | Ag(s) (aq) | C(s) Standard Hydrogen Electrode Under standard-state condition, the potential for the reduction of H+ at 25 C is taken to be EXACTLY ZERO. 2 H+ (1 M) + 2 e- H2 (1 atm) E = 0 V Think about Zn(s) | Zn2+ (1M) || Cu2+ (1M) | Cu (s) the measured emf =1.10 V at 25 C The redox reaction is spontaneous in the forward direction if the standard emf of the cell is positive. Example A galvanic cell consists of a Mg electrode in a 1.0 M Mg(NO3)2 solution and a Ag electrode in a 1.0 M AgNO3 solution. Calculate the standard emf of this electrochemical cell at 25 C. Standard Reduction Potential The voltage associated with a reduction reaction at an electrode when all solutes are 1 M and all gases are at 1atm. The hydrogen electrode serves as the reference for calculating the potential of a single electrode. -hydrogen gas is bubbled into a hydrochloric acid solution at 25 C; -the platinum electrode provides the surface and serves as an electrical conductor. Rules for using E 1. Applies to reactions as written. 2. The more positive E , the great tendency to be reduced (the stronger oxidizing agent). 3. The half-cell reactions are reversible and the sign of E will be changed. 4. Diagonal rule. 5. Multiplying reactions by some coefficient does not change E (intensive property – not affected by size of electrode or volume of electrolyte, but concentration must be 1M). Standard Reduction Potentials E cell = E reduction –E oxidation Modern voltmeters can read “+” or “–” volts. By always connecting the negative lead to SHE, the voltmeter reads E of the half-cell directly. 2 1/4/2010 Predicting Spontaneity Guess which half-cell is the anode. Write half-reactions accordingly. Calculate E cell. If E cell > 0, reaction is spontaneous (proceeds in the forward direction as written). 3