Document 14222990
advertisement

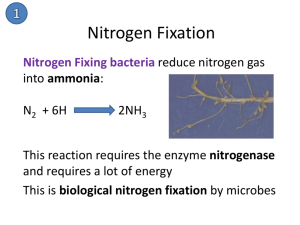
Background All life requires Nitrogen – – – Proteins Amino Acids Nucleic Acid 79% of the air is N2 – – This form cannot be used by most organisms Organisms need a fixed form (incorporated into a compound) Usable Nitrogen Plants secure Nitrogen in a fixed form – – nitrate ions (NO3−) ammonium (NH4+) Assimilation- Animals secure Nitrogen from plants and other animals that have eaten plants in organic material Nitrogen Fixation The breaking apart of N2 so that the atoms can combine with other atoms 3 processes: – – – 1) atmospheric fixation 2) biological fixation 3) industrial fixation Atmospheric Fixation Energy in lighting breaks apart N2 molecule and N combines with Oxygen in the air Nitrates (NO3-) forms in rainwater and falls to the earth 5-8% of the total nitrogen fixed Biological Fixation Some species of bacteria can fix atmospheric N2 into ammonium (NH4) Can be free living bacteria or symbiotically associated with plants Legumes form a symbiotic relationship with bacteria from the genus: Rhizobium Industrial Fixation Haber-Bosch Process Creates 500 million tons artificial fertilizer/year High pressure and intense heat (750-1200 degrees F) cause atmospheric Nitrogen and Hydrogen to form Ammonia (NH3) most of its is further processed to urea and ammonium nitrate (NH4NO3). Decomposition Proteins made by plants and animals pass through the food web Excretions and Dead animals get broken down by decomposing microorganisms Creates ammonia (NH3) Nitrification Ammonia from decay can be taken up directly by plants but usually converted first to nitrates (NO3-) Bacteria break down NH3 to nitrites (NO2−) and then to nitrates (NO3-). Denitrification Bacteria living deep in soils and aquatic Reduce Nitrates to Nitrogen gas (N2) so it returns to the atmosphere Can they keep up with the agricultural production? Eutrophication Excess Nitrates leach into ground water and get into bodies of water Algal blooms do well with the added nutrients (sometimes toxic) Prevent light from hitting deeper plants that produce O2, and their decomposition requires O2 use by the bacteria Reduced O2 in the water results Nitrates are harmful to humans and other organisms (amphibians)