Nitrogen-Cycle-info-cards
advertisement
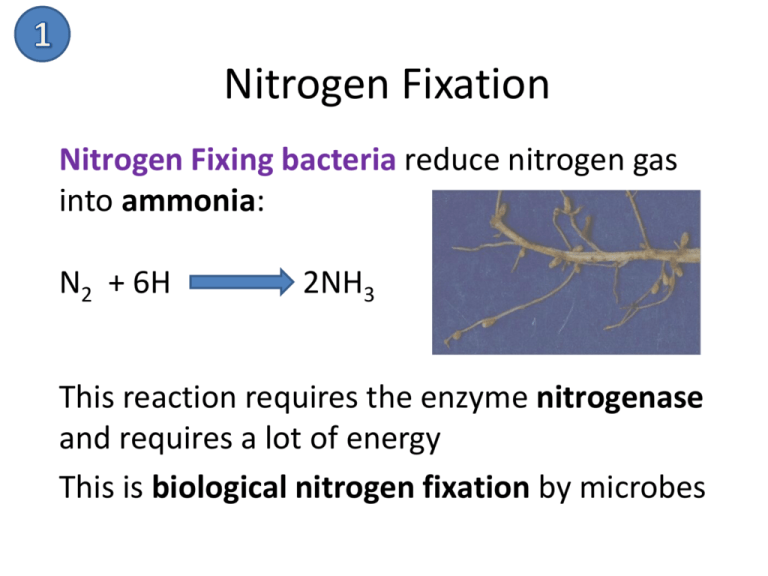
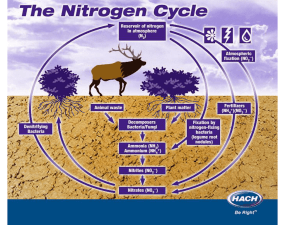
Nitrogen Fixation Nitrogen Fixing bacteria reduce nitrogen gas into ammonia: N2 + 6H 2NH3 This reaction requires the enzyme nitrogenase and requires a lot of energy This is biological nitrogen fixation by microbes Nitrogen Fixation Nitrogen fixation can also happen by industrial fixation. The Haber process produces ammonia from the reaction of nitrogen and hydrogen. Nitrogen Fixation Nitrogen fixation can also happen by atmospheric fixation by lightning. The energy in a lightning bolt can split nitrogen molecules in the air, allowing each nitrogen atom to react with oxygen to form nitrogen oxides. The rain washes these oxides to the ground, where they form nitrates. Nitrification • Ammonia can be taken up directly by plants usually through their roots. • However, most of the ammonia produced by decay is converted into nitrates. • This is accomplished in two steps: 1 type of nitrifying bacteria oxidise NH3 to nitrite (NO2-). • 2nd type of nitrifying bacteria convert the nitrites to nitrate (NO3-). Active Transport Nitrates are taken directly into a plant in uptake by the roots. ATP is used to open protein channels allowing nitrates, even at low concentrations, to be taken up via the process of active transport so that it can be used to make plant proteins. Ammonification Microbial saprobionts break down proteins in detritus to make ammonia: 1. Break down into amino acid using protease 2. Then remove amino groups from amino acids using deaminase Feeding Animals are able to gain proteins directly by consuming plant proteins. Denitrification Denitrifying bacteria convert nitrates into N2 and NOx gases This is a way that useful nitrogen is lost from the soil