Document 14194531
advertisement
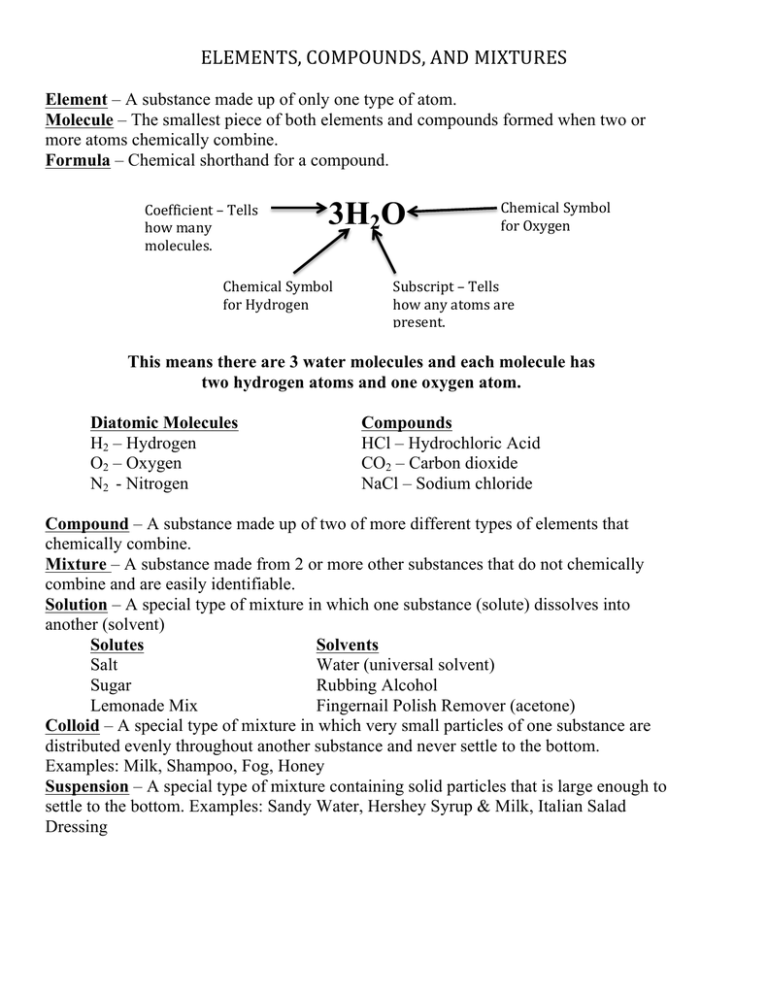
ELEMENTS, COMPOUNDS, AND MIXTURES Element – A substance made up of only one type of atom. Molecule – The smallest piece of both elements and compounds formed when two or more atoms chemically combine. Formula – Chemical shorthand for a compound. Coefficient – Tells how many molecules. 3H2O Chemical Symbol for Hydrogen Chemical Symbol for Oxygen Subscript – Tells how any atoms are present. This means there are 3 water molecules and each molecule has two hydrogen atoms and one oxygen atom. Diatomic Molecules H2 – Hydrogen O2 – Oxygen N2 - Nitrogen Compounds HCl – Hydrochloric Acid CO2 – Carbon dioxide NaCl – Sodium chloride Compound – A substance made up of two of more different types of elements that chemically combine. Mixture – A substance made from 2 or more other substances that do not chemically combine and are easily identifiable. Solution – A special type of mixture in which one substance (solute) dissolves into another (solvent) Solutes Solvents Salt Water (universal solvent) Sugar Rubbing Alcohol Lemonade Mix Fingernail Polish Remover (acetone) Colloid – A special type of mixture in which very small particles of one substance are distributed evenly throughout another substance and never settle to the bottom. Examples: Milk, Shampoo, Fog, Honey Suspension – A special type of mixture containing solid particles that is large enough to settle to the bottom. Examples: Sandy Water, Hershey Syrup & Milk, Italian Salad Dressing











