Bio Chem
advertisement

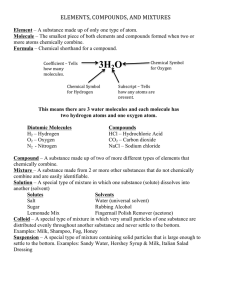
Brainstorm a list of factors that cause people to behave differently. Personality Experiences Heredity Environment Friends/Family Society WHY DO CELLS BEHAVE DIFFERENTLY? The combination of chemicals that form substances. •“ingredients” •“what an object is COMPOSED of” •Smallest unit of matter known. •100 million atoms lined up in a row = one centimeter •Composed of protons, neutron, electrons •A pure substance that consists of entirely one type of atom •Represented by letters or symbols •Approximately two dozen are found in living things •In total, 117 elements have been observed as of 2007, of which 94 occur naturally on Earth. Examples of elements O Oxygen H Hydrogen C Carbon Na Sodium Sulfur S N Nitrogen Potassium K Cl Chlorine A large percent Living things are made up of 4 elements: 1. Carbon (C) 2. Hydrogen (H) 3. Oxygen (O) 4. Nitrogen (N) Ex.) DNA molecule--> Ex: DNA Molecule Grey: carbon White: hydrogen Blue: Nitrogen Red: Oxygen Orange: phosphate How do I read the periodic table? Atomic number: # of protons There are an equal number of electrons as protons Atomic Mass: # of protons + # of neutrons •Substances that are formed by the chemical combination of two or more elements Most abundant compound on Earth is water ___________ •Elements have very different properties “actions/appearances” than the compounds that they form. H = Hydrogen = gas O = Oxygen = gas H2O = water = liquid • Are expressed through formulas 1. Chemical formulas 2.Structural formulas C12H22O11 C6H12O6 The attraction of atoms that cause element to form compounds All living things are made up of cells. Parts of the cell are made up of large complex molecules. Basic unit of most of these molecules is the element carbon (C). Carbon The carbon atom has four valence elections, this allows the carbon atom to form compounds with many other elements. C Examples of Carbon bonding Methane Other Carbon compounds •Carbon can form single, double or triple bonds. • Carbon can form a long chain or a ring structure. Identify the following as an elements or compounds: 1. Al 2. H2SO4 3. H2 7. Al2 8. S 9. NaCl 4. H2O 5. O2 6. NH4 10. C6H12O6 11. CO2 12. H There are two categories of compounds: •Organic •Inorganic •is any member of a large class of chemical compounds whose molecules contain carbon and hydrogen •Found in all living things Examples •C6H1206 •CH4 •Is any member of a large class of chemical compounds of molecules that contain earths elements •Not capable of creating life, no combination makes life Examples •NaCl • H2 O •CO2 Compounds Organic Contains Carbon & Hydrogen always together! Example: C6H12O6 glucose (sugar) Inorganic Contains the earth’s element Example: H2O pH is a scale designed to describe whether a compound is an acid or a base AND its strength. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Neutral Measures the concentration of H+ ions in a solution. BELOW 7, THE SUBSTANCE IS ACIDIC. ABOVE 7, THE SUBSTANCE IS BASIC. IF THE pH IS 7, THEN IT IS NEUTRAL. Using to test the level of pH Provides a specific number Used to identify is the substance in an acid or a base TASTE SOUR, REACT WITH METALS AND TURN LITMUS PAPER RED TASTE BITTER, FEEL SLIPPERY, REACT WITH LITMUS PAPER TO TURN IT BLUE Used to test for the presence of glucose Used to test for the presence of proteins Used to test for the presence of starch 1. Give examples of organic substances 2. Determine the pH of the following solutions.