Point of Care Testing - Oxygen Saturation - AVOX
advertisement

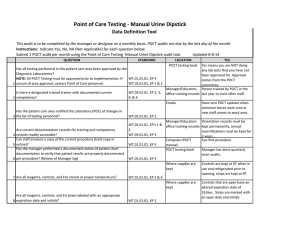
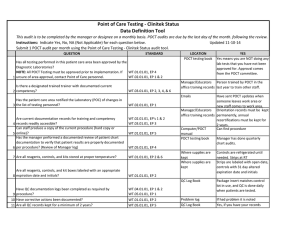
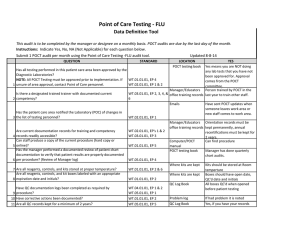
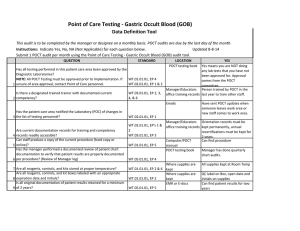
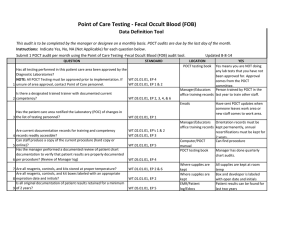
Point of Care Testing - Oxygen Saturation - AVOX Data Definition Tool This audit is to be completed by the manager or designee on a monthly basis. POCT audits are due by the last day of the month. Instructions: Indicate Yes, No, NA (Not Applicable) for each question below. Submit 1 POCT audit per month using the Point of Care Testing -Oxygen Saturation (AVOX) audit tool. Updated 11-10-14 QUESTION STANDARD Has all testing performed in this patient care area been approved by the Diagnostic Laboratories? NOTE: All POCT Testing must be approved prior to implementation. If WT.01.01.01, EP 4 WT.02.01.01, EP 1 & 2 1 unsure of area approval, contact Point of Care personnel. Is there a designated trained trainer with documented current 2 competency? WT.03.01.01, EP 2, 3, 4, &6 LOCATION POCT testing book YES Yes means you are NOT doing any lab tests that you have not been approved for. Approval comes from the POCT committee. Manager/Educators Person trained by POCT in the office training records last year to train other staff. Emails Has the patient care area notified the Laboratory (POC) of changes in 3 the list of testing personnel? Are current documentation records for training and competency 4 records readily accessible? Can staff produce a copy of the current procedure (hard copy or 5 online)? Has the manager performed a documented review of patient chart documentation to verify that patient results are properly documented 6 per procedure? (Review of Manager log) 7 Are all reagents, controls, and kits stored at proper temperature? WT.02.01.01, EP 1 WT.02.01.01, EP's 1 & 2 WT.03.01.01, EP 3 WT.01.01.01, EP 5 Manager/Educators Orientation records must be office training records kept permanently, annual recertifications must be kept for 2 years. Computer/POCT Can find procedure manual POCT testing book Manager has done quarterly chart audits. WT.05.01.01, EP 4 Where supplies are kept WT.01.01.01, EP 2 & 6 Where supplies are kept Are all reagents, controls, and kit boxes labeled with an appropriate 8 expiration date and initials? Have sent POCT updates when someone leaves work area or new staff comes to work area. WT.01.01.01, EP 2 Controls are kept at RT for 12 months or refrigerated, cartridges are kept at RT Controls are labeled with 1 hour altered exp and initials when reconstitued. Labeled with 12 month altered date once removed from refrigerator Have QC documentation logs been completed as required by 9 procedure? 10 Have corrective actions been documented? 11 Are all QC records kept for a minimum of 2 years? QC is run weekly, on all new lots or shipments of cartridges, Optical QC is done daily Problem log QC Log Book QC Log Book If had problem it is noted Yes, if you have your records There is a signature to indicate reviewed weekly. patient log book or Edocs POCT testing book Are they accessible for last 2 years All CAP proficiency samples have been run and results returned to POCT Optical filters are checked WT.04.01.01, EP 1 & 2 WT.05.01.01, EP 1 WT.01.01.01, EP 2 WT.05.01.01, EP 5 WT.01.01.01, EP 2 Has the manager or designee performed a weekly review and sign off of WT.04.01.01, EP 1 & 2 WT.05.01.01, EP 1 12 quality control and other test specific required documentation? Is all original documentation of patient results retained for a minimum 13 of 2 years? WT.05.01.01, EP 5 Where appropriate, has proficiency testing been performed per 14 procedure, reported within deadlines? 15 Are maintenance records available and complete? QC Log Book WT.03.01.01, EP 1, 4, & 5 WT.05.01.01, EP 4 & 5 Maintenance Log