Point of Care Testing - MONO Data Definition Tool
advertisement

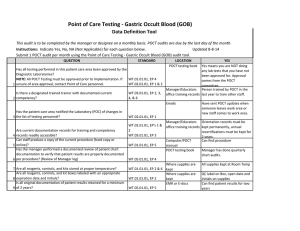
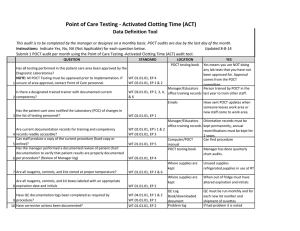
Point of Care Testing - MONO Data Definition Tool This audit is to be completed by the manager or designee on a monthly basis. POCT audits are due by the last day of the month. Instructions: Indicate Yes, No, NA (Not Applicable) for each question below. Submit 1 POCT audit per month using the Point of Care Testing -MONO audit tool. Updated 8-8-14 QUESTION 1 2 3 4 5 6 7 8 9 10 11 STANDARD LOCATION POCT testing book YES Yes means you are NOT doing Has all testing performed in this patient care area been approved by the any lab tests that you have not Diagnostic Laboratories? been approved for. Approval NOTE: All POCT Testing must be approved prior to implementation. If WT.01.01.01, EP 4 comes from the POCT unsure of area approval, contact Point of Care personnel. WT.02.01.01, EP 1 & 2 committee. Manager/Educators Person trained by POCT in the Is there a designated trained trainer with documented current WT.03.01.01, EP 2, 3, 4, office training records last year to train other staff. competency? &6 Emails Have sent POCT updates when someone leaves work area or Has the patient care area notified the Laboratory (POC) of changes in new staff comes to work area. the list of testing personnel? WT.02.01.01, EP 1 Manager/Educators Orientation records must be office training records kept permanently, annual Are current documentation records for training and competency WT.02.01.01, EP's 1 & 2 recertifications must be kept for records readily accessible? WT.03.01.01, EP 3 2 years. Can staff produce a copy of the current procedure (hard copy or Computer/POCT Can find procedure online)? WT.01.01.01, EP 5 manual Has the manager performed a documented review of patient chart POCT testing book Manager has done quarterly documentation to verify that patient results are properly documented chart audits. per procedure? (Review of Manager log) WT.05.01.01, EP 4 Where supplies are Kits are stored at Room Are all reagents, controls, and kits stored at proper temperature? WT.01.01.01, EP 2 & 6 kept Temperature Where supplies are All boxes are labeled with box Are all reagents, controls, and kit boxes labeled with an appropriate kept number, date received, date expiration date and initials? WT.01.01.01, EP 2 QC'd and initials QC Log Book QC is noted for all opened boxes Have QC documentation logs been completed as required by WT.04.01.01, EP 1 & 2 of test kits procedure? WT.05.01.01, EP 1 Problem log If had problem it is noted Have corrective actions been documented? WT.01.01.01, EP 2 QC Log Book Yes, if you have your records Are all QC records kept for a minimum of 2 years? WT.05.01.01, EP 5 WT.01.01.01, EP 2 Has the manager or designee performed a weekly review and sign off of WT.04.01.01, EP 1 & 2 WT.05.01.01, EP 1 12 quality control and other test specific required documentation? Is all original documentation of patient results retained for a minimum 13 of 2 years? WT.05.01.01, EP 5 QC Log Book There is a signature to indicate reviewed weekly. Patient Log book/Edocs Are they accessible for last 2 years