Developmental reorganisation of visual motion pathways
advertisement

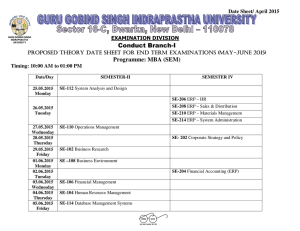
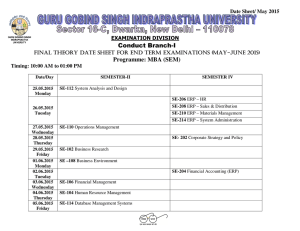
Developmental reorganisation of visual motion pathways John Wattam-Bell, Melissa Chiu & Louisa Kulke Visual Development Unit, Dept. of Developmental Science, Division of Psychology & Language Sciences, UCL Introduction Results 2: motion onset ERP Globally coherent visual motion activates a network of extrastriate cortical areas, including V5/MT+, V3a and V61. Little is known about the development of this network. The cortical activations displayed on the right show absolute values of ERP source strength Adult subjects Recent evidence from steady-state event-related potentials (ERPs) to motion coherence indicates a substantial reorganisation of the network between infancy and adulthood2. Methods 0 −2 −4 ERP components were defined as the space-time connected regions of positive or negative scalp potential that emerge from thresholding the signal above and below the baseline3. Component significance was assessed with a randomization test3. Unless otherwise stated, the components discussed here were significant at p<0.05, corrected for FWE. Brainstorm7 was used to estimate the cortical sources of the ERPs, based on nominal (ie not measured) electrode positions, coregistered to a standard adult head (MNI Colin27). Results 1: motion coherence ERP The coherence ERP is the differential response to the two levels of coherence - ie a global response, requiring integration of local motion information. Topographic plots of scalp potential at indicated times. Front of head at top. Red = positive blue = negative. 1.5 1 0.5 0 −0.5 −1 100 150 200 250 300 time (msec) 350 400 450 500 150 200 250 300 time (msec) 350 400 450 500 Group average ERP across all channels, from stimulus onset at time=0 Posterior ERP components labelled in the figure above: A: bilateral negative components from ~160 msec, consistent with V5/MT+ activation B: midline positive component from ~200 msec, consistent with a V1/V2 origin. Feedforward responses in V1/V2 are not expected to show differential sensitivity to coherence, which is a global stimulus property. Global responses in these areas are probably driven by feedback from extrastriate areas4. The relative timing of the V5/MT+ and V1/V2 ERP components is consistent with this. Infant subjects The infants showed no real evidence of a coherence response. This is probably due to the low signal-to-noise ratio of transient ERPs; the more sensitive steady-state technique gives clear evidence for differential responses to motion coherence at these ages3. A: 118 msec B B: 398 msec 10 0 50 100 150 200 250 300 time (msec) 350 400 450 500 Starts similarly to adult ERP (A: V1/V2, spreading laterally), but no abrupt switch. Instead, the spread continues to distinct lateral foci (B) with sources in V5/MT+. This infant ERP may reflect purely forward activation, without the feedback from extrastriate cortex to V1/V2 that seems to dominate the ERP in adults. Infants 6-9 months C C: 278 msec amplitude (microvolts) Group analysis • The individual coherent and incoherent averages were combined in two ways: ▪ motion coherence ERP = coherent response - incoherent response. Differential response to the two levels of coherence, 0% & 100%. ▪ motion onset ERP = coherent response + incoherent response. Response to onset of motion in an initially static pattern. B 100 20 −10 0 coherent motion trial Individual data inter-trial • filtering: 0.2 - 30 Hz (3rd order zero-phase filters) interval • epoching: -200 to 800 msec relative to trial onsets • artifact rejection, baseline correction, average reference • epoch averaging: separate averages for coherent and incoherent motion trials A 50 A amplitude (microvolts) inter-trial interval Adult subjects C: 398 msec Infants 3-5 months incoherent motion trial ERP processing and analysis amplitude (microvolts) 2 B: negative component from V1/V2 which dominates the rest of the ERP (C). This component may reflect feedback from extrastriate cortex to V1/V2 Stimulus • random-dot kinematogram, 2000 dots, dot size 0.2 deg, area 35 x 26 deg • coherent motion: dots moved along circular trajectories at 6.1 deg/sec (linear speed) • incoherent motion: dots moved in random directions at 6.1 deg/sec 50 4 A: positive component (V1/V2), spreading laterally (V3a?) before switching at 180 msec to Subjects • 13 adults, aged 18-36 years • 21 infants; infants were tested twice, first at 3-5 months of age, then at 6-9 months ERP trials • moving stimulus presented in 0.8 sec trials • random inter-trial interval (1.2 - 3 sec) with static dots • coherent and incoherent motion trials randomly interleaved A: 158 msec C 6 0 ERPs were recorded with a 128-channel EGI geodesic system 0 amplitude (microvolts) Steady-state ERPs give a sensitive measure of cortical responses, but confound timing and polarity information that could shed light on the nature of the reorganisation. To address this, we have measured transient ERPs to the onset of coherent and incoherent motion in infants and adults. The results suggest that emergence of feedback from extrastriate cortex to V1/V2 may play a key role in the developmental reorganisation of the motion pathways. B A 15 10 5 0 −5 −10 0 50 100 150 200 250 300 time (msec) 350 400 450 500 The 6-9 month ERP is very similar to that at 3-5 months. However, there is an additional (but not significant: p=0.09) negative midline component (C), as seen in adults but not at 35 months – the first hint of emerging feedback to V1/V2? Discussion In the mature visual system, cortical feedback produces contextual (extra-classical) effects4, allowing global information to modulate activity in local areas such as V1/V2. This feedback probably underlies the late onset V1/V2 components that dominate the adult ERP. The absence of these components in younger infants suggests that emergence of cortical feedback might be a major factor in the developmental reorganisation of cortical motion processing. This is consistent with the well-known pattern of rapid proliferation of cortical synapses in the first months of life, followed by gradual pruning throughout childhood5. This pattern is largely confined to lateral and feedback connections6, which implies that these connections are the main site of developmental cortical plasticity. During the phase of synaptic proliferation, newly-formed connections are presumably quite random, with limited functionality; it is probably the subsequent pruning that imposes functional order. Interestingly, the transition between these two phases occurs at about 7-8 months, around the time when the first hint of feedback is seen in our ERP data. References 1. Braddick et al (2001) Perception 30, 61; Pitzalis et al (2010) Cerebral Cortex 20, 411 2. Wattam-Bell et al (2010) Current Biology 20,411 3. Maris & Oostenveld (2007) J. Neurosci. Methods 164, 177 4. Stillito et al (2006) TINS 29, 307 5. Huttenlocher (1982) Neuroscience Letters 33,247 6. Burkhalter (1993) Cerebral Cortex 3, 476 7. Tadel et al (2011) Computational Intelligence and Neuroscience, vol. 2011, ID 879716