Organic LEDs – part 6
advertisement
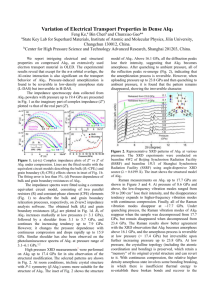
Organic LEDs – part 6 • Exciton Recombination Region in Organic LEDs • White OLED • Flexible OLEDs • Solvation Effect • Solid State Solvation Handout: Bulovic, et al., Chem. Phys. Lett. 287, 455 (1998); 308, 317 (1999). @ MIT April 17, 2003 – Organic Optoelectronics - Lecture 19 1 Exciton can transfer its energy to: Bulović ´ et al., Phys. Rev. B 58, 3730 (1998). RADIATIVE modes: SURFACE emission * radiation modes * waveguide modes NON-RADIATIVE modes: * internal conversion, intersystem crossing * surface plasmons * losses due to absorption in metal electode RADIATION Modes Glass Θ k EDGE emission ITO WAVEGUIDE Modes α - NPD Alq3 Glass Substrate OLED - EL Region Metal Losses Surface Plasmons Mg:Ag Electrode 2 ExternaL EL Quantum and Power Efficiency (ηR and ηP) for ITO/ 350Å NPD/ xÅ Alq / Mg:Ag / Ag ηR (SURFACE) [%] 0 500 1000 1500 0.6 0.4 0.2 200 0.6 150 0.4 100 0.2 50 0.0 0 500 1000 0 1500 Power Consumed (@10mA/cm2) [mW/cm2] ηP (SURFACE) (@10mA/cm2) [lm/W] 0.0 Alq3 Thickness [Å] 3 PL Dependence on Alq3 Thickness Bulović ´ et al., Phys. Rev. B 58, 3730 (1998). PL 540 PL Peak [nm] Normalized Intensity 1.0 0.8 NO ELECTRODE 530 ELECTRODE 0.6 520 0.4 400 600 800 Alq3 Thickness [Å] 300 Å Alq3 600 Å Alq3 Θ = 0º 800 Å Alq3 0.2 0.0 450 200 500 550 600 650 700 750 Wavelength [nm] 4 EL Dependence on Alq3 Thickness Bulović ´ et al., Phys. Rev. B 58, 3730 (1998). 550 EL EL Peak [nm] Normalized Intensity 1.0 0.8 THEORY 540 530 0.6 200 0.4 400 500 Alq3 Thickness [Å] 200 Å Alq3 350 Å Alq3 Θ = 0º 500 Å Alq3 0.2 300 Theoretical Fit (to 200 Å Alq3 spectrum) 0.0 450 500 550 600 650 700 750 Wavelength [nm] 5 Top to Side EL Ratio - Dependence on Alq3 Thickness Bulović ´ et al., Phys. Rev. B 58, 3730 (1998). ΦSURFACE / ΦEDGE 2.0 Experiment Theory 1.5 1.0 SURFACE Detector EDGE Detector 0.5 OLED Glass Substrate 0.0 100 200 300 400 500 600 700 Alq3 Thickness [Å] 6 EL Recombination Region Dependence on Current Bulović ´ et al., Phys. Rev. B 58, 3730 (1998). LREC-1 [nm-1] 0.05 0.03 0.01 0 0.06 1.62 (a) (b) 0.05 0.04 1.58 0.03 1.56 0.02 1.54 0.01 1.52 1.50 10-4 LREC-1 [nm-1] ΦSURFACE / ΦEDGE 1.60 0.00 10-3 10-2 Current [A/cm2] 10-4 10-3 10-2 Current [A/cm2] 7 depth EL Recombination Region Dependence on Current Cathode increasing current Alq3 (35 nm) RF 2% DCM2 in Alq3 (5nm) J [mA/cm2] DCM2 1300 130 13 1.3 0.13 TPD (40 nm) 100 EL Fraction [%] Normalized Intensity exciton density 80 DCM EL 60 Glass 40 Alq3 EL 20 0 -1 0 Alq3 400 Anode 1 2 3 log(J[mA/cm2]) 500 600 700 Wavelength [nm] 800 Coe et al., Org. Elect. (2003) 8 Wavelength [nm] Tuning Emission of White OLEDs 500 600 700 1.0 0.6% 1.5% 3% 6% 0.8 Intensity [a.u.] Ag Mg:Ag 400 Alq3 BCP 0.6 changing DCM2 in α-NPD concentration (with 40A BCP) 0.4 0.2 NPD 0.0 NPD:DCM2 1.0 BCP – exciton blocking layer 0.8 Intensity [a.u.] glass DCM2 120 Å BCP 80 Å BCP 40 Å BCP TPD ITO Alq3 White OLED (0.33,0.33) 0.6 changing BCP layer thickness 0.4 (with 0.6% DCM2) 0.2 0.0 400 500 600 Wavelength [nm] 700 9 Flexible OLED (FOLED) - Ultra lightweight Thin form factor Rugged Impact resistant Conformable Manufacturing Paradigm Shift Web-Based Processing 10 FOLED-based Pixelated, Monochrome Display Source: UDC, Inc. 11 Transparent FOLED-based Pixel Source: UDC, Inc. 12 Packaging of OLEDs – Multilayer Coatings Barix is a coating composed of alternating layers of polymer and ceramic thin films that can be deposited on a plastic substrate or directly on an OLED display. The technology breakthrough that enables this to be used as the packaging material for flat panel displays is the creation of a barrier layer 10,000 times better than anything currently produced. From Vitex, makers of barix coating 13 The PRESENT … … and the nearby FUTURE … … of ORGANIC DISPLAY TECHNOLOGY 14 Luminescence of Molecules in Solid Matrices • Solid State Solvation (SSS) • • Time-Resolved SSS • @ MIT 15 Electroluminescence in Doped Organic Films 1. 2.6 eV Excitons formed from combination of electrons and holes transparent anode electrons 2.7 eV trap states a-NPD low work function cathode Alq3 exciton holes 5.7eV 6.0 eV host molecules (charge transport material) 2. Excitons transfer to luminescent dye dopant molecule (luminescent dye) 16 Effect of Dopants on the OLED EL Spectrum 1.0 N Normalized EL Intensity O 0.8 0.6 Al N O Alq3 NC 3 CN DCM2:Alq3 PtOEP:Alq3 0.4 N N Pt N N 0.2 0.0 400 500 600 700 800 Wavelength [nm] 17 Electroluminescence of x% DCM2 in Alq3 OLEDs tuning range 35 nm Intensity [a.u.] 1.0 0.5 % 1.5 % 2.5 % 4.5 % 6% 0.8 0.6 0.4 Alq3 0.2 0.0 400 500 600 700 800 Wavelength [nm] 18 Alq3 DCM2 in Alq3 low DCM2 high DCM2 19 Solvatochromism - Historical Perspective It has been long recognized that UV/visible absorption spectra can be influenced by: - THE PHASE (gas or liquid) - SOLVENT 1878 - Kundt established relationship between solvent effect and solvent properties (Kundt’s Rule) using chlorophyl, fuchsin, aniline green, cyanine, quinizarine, and egg yolk, in twelve different solvents he concluded that increasing index of refraction of the solvent is related to the red-shift in the absorption spectrum of the solute 20 ... change in the spectral position of aborption/luminescence band due to change in the polarity of the medium dipolar molecule dipolar lumophore dipole - dipole interaction modifies the energy structure of the molecules Ö solvation is a physical perturbation of lumophore’s molecular states Ö isolated molecule (in a gas phase) and solvated molecule are in the same chemical state (no solvent induced proton or electron transfer, ionization, complexation, isomerization) 21