Anomalous Variation of Electrical Transport Properties in
advertisement

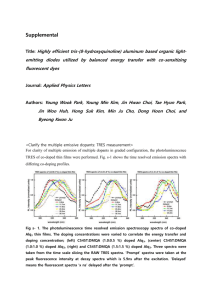
Variation of Electrical Transport Properties in Dense Alq3 Feng Ke,a Bin Chenb and Chunxiao Gao*a a State Key Lab for Superhard Materials, Institute of Atomic and Molecular Physics, Jilin University, Changchun 130012, China. b Center for High Pressure Science and Technology Advanced Research, Shanghai 201203, China. We report intriguing electrical and structural properties on compressed Alq3, an extensively used electron transport material in OLED. The experimental results reveal that except for the π-π orbital overlaps, the Al-oxine interaction is also significant on the transport behavior of Alq3. Pressure-induced amorphization is found to be reversible in low-density amorphous state (L-DAS) but irreversible in H-DAS. The impedance spectroscopy data collected from Alq3 powders with pressure up to 19.4 GPa are presented in Fig. 1 as the imaginary part of complex impedance (Z″) plotted vs that of the real part (Z′). Figure 1. (a)-(c) Complex impedance plots of Z″ vs Z′ of Alq3 under compression. Lines are the fitted results with the equivalent circuit models describing the bulk (R1-CPE1) and grain boundary (R2-CPE2) effects shown in inset of Fig. 1b. The fitting error is less than 5%. (d) Pressure dependence of bulk and grain boundary resistances of Alq3. The impedance spectra were fitted using a common equivalent circuit model, consisting of two parallel resistors (R) and constant-phase element (CPE) elements (Fig. 1) to describe the bulk and grain boundary relaxation processes, respectively, on Zview2 impedance analysis software. The obtained bulk (Rb) and grain boundary resistances (Rgb) are plotted in Fig. 1d. Rb of Alq3 increases markedly at low pressures (< 3.1 GPa), followed by a shoulder from 3.1 to 5.7 GPa, and continues the increasing tendency up to 7.9 GPa. However, it changes the pressure dependence with continuous compression and drops rapidly up to 15.9 GPa. Similar shoulder has also been observed on the photoluminescence spectra of Alq3 at pressure range of 3.1~6.1 GPa.1,2 High pressure XRD measurements7 were performed on Alq3 up to 17.4 GPa for in situ observation of the structural modification. The selected patterns are shown in Fig. 2. At room conditions, triclinic crystal structure with P-1 symmetry (β-Alq3) seems more suitable for the structure of Alq3. The inset of Fig. 2 shows the structure model of Alq3. Above 16.1 GPa, all the diffraction peaks lose their intensity, suggesting that Alq3 becomes amorphous. After quenching to ambient pressure, all of the reflection peaks re-emerge (Fig. 2), indicating that the amorphization process is reversible. However, when uploading pressure up to 23.8 GPa and then quenching to ambient pressure, it is found that the pattern remains disappeared, showing the irreversible character. Figure 2. Representative XRD patterns of Alq3 at various pressures. The XRD experiments were conducted on beamline 4W2 of Beijing Synchrotron Radiation Facility (BSRF) and beamline 15U1 of Shanghai Synchrotron Radiation Facility (SSRF) using angle-dispersive XRD source (λ = 0.6199 Å). The inset shows the structural model of Alq3. Raman measurements on Alq3 up to 17.7 GPa are shown in Figure 3 and 4. At pressure of 8.6 GPa and above, the low-frequency vibration modes ranged from 50 to 200 cm-1 lose their intensity, and the disappearance tendency expands to higher-frequency vibration modes with continuous compression. Finally, all of the Raman vibration modes disappear at ~17.7 GPa. Under quenching process, the Raman vibration modes of Alq3 reappear when the sample was decompressed from 17.7 GPa, but remain disappeared when decompressed from 23.4 GPa. The Raman results show better agreement with the XRD observation that Alq3 becomes amorphous above 16.1 GPa, and the amorphous process is reversible at low pressure (< 17.4 GPa) but irreversible upon further increasing pressure up to 23.8 GPa. At low pressure, the crystalline topology (including the atomic coordination and bonding) is preserved, which retain a “memory” of its original crystal structure and can revert to it. With continuous compression, the relative higher density amorphous state involves some bonding breaking, in which there is insufficient thermal energy to re-establish these broken bonds and recover to the original state, and consequently the pressure-induced amorphization will be irreversible. Figure 3. High-pressure Raman spectra of Alq3 with the Raman peaks of diamond at ~1331 cm-1 subtracted. Here the def., wag., str., and bre. represent the deformation, wagging, stretching, and breathing vibration modes, respectively. As clearly seen from Fig. 4, the Raman active modes lose their intensity gradually from low- to high-frequency above 8.0 GPa, that is the Al-oxine bonds are firstly tuned, and then the oxine ligands lose their long-term ordering, followed by the modification of the ring and C-H bonds with increasing pressure. The relative Raman intensity changes of the Al-oxine vibration modes (Fig. 4) should be caused by the pressure-induced modification of the Al-oxine bonds and the non-planar conformation of the Alq3 molecules. Figure 4. (a) Pressure dependence of Raman peaks positions of Alq3. (b) and (c) The ratio of the intensity of the ~65 cm-1, ~90 cm-1, and ~170 cm-1 Raman vibration modes to the ~1400 cm-1 mode in Alq3 as a function of pressure. (d) The variation of the Raman peak width (~1400 cm-1) under pressure. Under compression, the π-π interaction of Alq3 is greatly enhanced, which narrows the LUMO and HOMO gap,3 and hence will facilitate the electrical transport of the π-π orbital interaction. That is the conduction of Alq3 should be improved under compression, which is not consistent with the behavior of Rb that increases substantially within pressure range of 1.6~7.9 GPa. It implies that in addition to the significance of the π-π interaction on the transport properties of Alq3, some other factors play important roles as well. The similar change tendency between the Al-oxine deformation mode (at ~90 cm-1, shown in Fig. 4) and Rb below 8.0 GPa easily reminds one of their correlation. At ambient pressure, resulting from the low symmetry of crystal structure that those three Al-oxine ligands (including the Al-O and Al-N bonds) each have different bond lengths and angles, the charges are not localized uniformly on three ligands.3b Such conformation makes the Al-oxine interaction sensitive with pressure, and causes the pronounced enhancement of the Al-oxine deformation modes, which describe the variation of the bond angles and torsion angles. The strengthened Al-oxine bonds, especially the Al-N and Al-O bonds, should localize the charge carriers, and hence hinder the conduction of Alq3. Moreover, the Al-oxine bonds angle changes can result in defects and increase the degree of structural disorder, accounting for the broadening in the Raman spectra and XRD patterns of Alq3 under compression, which can also capture the charge carriers and limit the electrical transport. Thus, Rb of Alq3 increases with uploading pressure. At higher pressure (>8.0 GPa), the essential changes of Al-oxine interaction fundamentally modify the electronic structure of Alq3, driving the redistribution of electron density between adjacent oxine ligands and the localized-delocalized charger states transfer, which combine with the improved conduction caused by enhanced π-π orbital overlaps and non-planar to more-planar conformation modification under compression compensate the hindering effect of structural disorder or defects, and cause Alq3 better conductive. The dramatic increase of Rb above 16.4 GPa should be induced by the amorphization process, which bring about a higher degree of structural disorder and kill the charge carrier of Alq3. It suggests that the Al-oxine interaction also play an important role on the electrical transport properties of Alq3 under compression. References: 1. I. Hernandez and W. P. Gillin, J. Phys. Chem. B 2009, 113, 14079; 2. I. Hernandez, W. P. Gillin and M. Somerton, J. Lumin. 2009, 129, 1835. 3. F. F. Muhammad, A. I. A. Hapip and K. Sulaiman, J. Organomet. Chem. 2010, 695, 2526;