The ecology of selected grasshopper species along an elevational gradient
advertisement

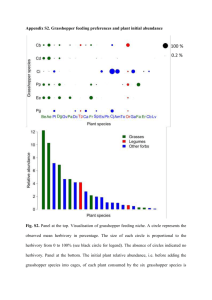
The ecology of selected grasshopper species along an elevational gradient by David Harrison Wachter A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Entomology Montana State University © Copyright by David Harrison Wachter (1995) Abstract: The extent to which grasshopper communities along an elevational gradient change in southwest Montana was studied. In addition to this, four species were chosen to examine changes in phenology along the gradient. This thesis examines the hypothesis that species assemblages change with elevation and that intraspecific variation in phenology occurs with changes in elevation. I used detrended correspondence analysis to evaluate changes in grasshopper and plant species across eleven sampling sites of varying elevation and aspect. Weekly sampling of each site allowed for the plotting of phenologies for Melanoplus sanguinipes, Melanoplus bivittatus, Chorthippus curtipennis, and Melanoplus oregonensis. The results showed non-random distribution of grasshopper species along the gradient. Grasshopper species distribution was significantly correlated to elevation, precipitation, proportion of grasses and forbs, and the total number of plant species. Phenological studies showed thatnymphal emergence was later at sites greater in elevation. Adults from higher elevation sites appeared at collection dates nearly equal to those from lower elevation sites. THE ECOLOGY OF SELECTED GRASSHOPPER SPECIES ALONG AN ELEVATIONAL GRADIENT by David Harrison Wachter A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Entomology MONTANA STATE UNIVERSITY Bozeman, Montana December 1995 Uii+* APPROVAL of a thesis submitted by David Harrison Wachter This thesis has been read by each member of the thesis committee and has been found to be satisfactory regarding content, English usage, format, citations, bibliographic style, and consistency, and is ready for submission to the College of Graduate Studies. Approved for the Major Department Date Head, M^joi Approved for the College of Graduate Studies LL ___ Date ' Graduate De; STATEMENT OF PERMISSION TO USE In presenting this thesis in partial fulfillment of the requirements for a master’s degree at Montana State University, I agree that the Library shall make it available to borrowers under rules of the Library. If I have indicated my intention to copyright this thesis by including a copyright notice page, copying is allowable only for scholarly purposes, consistent with 6Tair use” as prescribed in the U.S. Copyright Law. Requests for permission for extended quotation from or reproduction of this thesis in whole or parts may be granted only by the copyright holder. Signature Date____ ACKNOWLEDGEMENTS I express my sincere thanks to.my advisor, Kevin O’Neill, for his support, guidance, and patience throughout this project. Thanks also to Bill Kemp, Matt Lavin, and Bob Nowierski for their support and insightful reviews of the thesis. I wish to acknowledge the Center for Mountain Studies at Montana State University for their support and the classes they provided through which I gained valuable insight into the uniqueness of mountain environments. I also wish to acknowledge the U.S. Forest Service for allowing me to maintain weather stations on land in the Gallatin National Forest. My sincere thanks go to all Entomology faculty and staff for all their help. Lastly, thanks to my family for their never ending love and support. V TABLE OF CONTENTS. Page APPROVAL ...................................................................................................................... ii STATEMENT OF PERMISSION TO USE ............................................................... iii ACKNOW LEDGEM ENTS ........................................... TABLE OF CONTENTS ......... iv v LIST OF TABLES .................................................... vii LIST OF FIGURES ........................................................................... vii A BSTRACT ....................... ix 1. INTRODUCTION ........................................................................................................ I Importance of grasshopper studies ................................................................... I Climate of southwestern Montana ........................................... 2 Vegetation of southwestern Montana ........................................ 3 Grasshoppers in steppe and mountain environments ...................................... 4 Distribution o f Acrididae ................................................................................ 5 Phenology o f Acrididae .............. — ........ ....................... ................................ 8 Objectives of distribution and phenology studies .......................................... 10 2. MATERIALS AND METHODS ................................ 11 Study Sites ........................................................................................................ 11 Sam pling Techniques ............ 12 Grasshoppers Plants ............................................................. ......................................................................................................... 12 13 Temperature Data and Phenology ................................................................... 13 3. Identification of Acrididae .................. 15 Data A nalysis ...................................................................... 16 RESULTS ........................................................ '........................................................ 17 vi TABLE OF CONTENTS (cont.) Species Distribution ........................................................................................... Grasshoppers 17 ......................................................................................... 17 Grasshopper DCA Results ...................................... 22 Plants 25 ........................................................................................ Plant DCA Results ................................................................................ 29 Grasshopper phenology .......... 4. 31 Melanoplus sahguinipes ................... 31 Melanoplus bivittatus ..................................................................... 33 Chorthippus curtipennis ........ 36 Melanoplus oregonensis ......................................................................... 36 DISCUSSIO N ........................................................................... 41 Summary of patterns of grasshopper and plant distribution.............................41 Environmental correlates of grasshopper distribution......................................42 Summary o f patterns of grasshopper development ...................................... 47 Environmental correlates of grasshopper developm ent.....................................48 BIBLIOGRAPHY ............................................................................................................ 52 vii LIST OF TABLES Table Page 1. Grasshopper species collected ................................................................... 18 2. Julian dates for the first collection of second instars ................................. 23 3. Correlation computed for comparisons between DCA axes scores and environmental variables .............. 26 4. Plant species collected and used for DCA analysis........ ......................... 28 A. viii LIST OF HGURES Figure Page 1. Number of species as a function of e lev a tio n .......................................... 19 2. Number of individuals collected from each sampling s it e ..........................20 3. Detrended correspondence analysis of 11 grasshopper sampling sites across 4 vegetation zones .............. ............. ................. ............................. 24 4. Detrended correspondence analysis of 25 grasshopper species for 11 sampling sites across 4 vegetation zones .................;......................... 27 5. Detrended correspondence analysis of 11 plant communities across 4 vegetation zones .............................................................................. 30 6. M. sanguinipes development as a function o f Julian d a te .......................... 32 7. Mean instar of M. sanguinipes plotted against accumulated degree days......34 8. Plots of Melanoplus bivittatus development presented as mean instar as a function o f Julian date ....................................................................... 35 9. Plots of C. curtipennis development presented as mean instar as a function of Julian date .............................. 37 10. Plots of M. oregonensis development presented as mean instar as a function of Julian date ................................ 38 11. Mean instar of M. oregohensis plotted against accumulated degree-days.... 40 ABSTRACT The extent to which grasshopper communities along an elevational gradient change in southwest Montana was studied. In addition to this, four species were chosen to examine changes in phenology along the gradient. This thesis examines the hypothesis that species assemblages change with elevation and that intraspecific variation in phenology occurs with changes in elevation. I used detrended correspondence analysis to evaluate changes in grasshopper and plant species across eleven sampling sites of varying elevation and aspect. Weekly sampling of each site allowed for the plotting of phenologies for Melanoplus sanguinipes, Melanoplus bivittatus, Chorthippus curtipennis, and Melanoplus oregonensis. The results showed non-random distribution of grasshopper species along the gradient. Grasshopper species distribution was significantly correlated to elevation, precipitation, proportion of grasses and forbs, and the total number of plant species. Phenological studies showed thatnymphal emergence was later at sites greater in elevation. Adults from higher elevation sites appeared at collection dates nearly equal to those from lower elevation sites. I INTRODUCTION Importance of Grasshopper Studies Southwest Montana is composed of regions of steppe and mountain ranges (Price, 1981). Grasslands within these regions are distributed within a complex topography. This regional variation creates environmental gradients that affect plant and animal assemblages at the landscape and community scale (Senft et al. 1987). These gradients also provide ecologists with opportunities to study the spatial and temporal aspects of insect diversity and abundance. In the temperate grasslands of North America, grasshoppers (Orthoptera: Acndidae) are among the most abundant and economically important grazing herbivores (Otte 1981). Their abundance and food preferences occasionally make them strong competitors with humans because they consume food crops and livestock forage. For this reason, they are a relatively well-studied group (Alexander and Hilliard 1969, Evans 1988, Joem 1979, 1982, Kemp et al. 1989,1990, Uvarov, 1931). However, researchers have only recently addressed the factors that influence grasshopper distribution (Evans 1987, Kemp et al, 1990a,b). In this thesis, I present the results from studies of grasshopper distribution along an environmental gradient from the steppe regions in the Gallatin Valley of southwest Montana to alpine regions of surrounding mountain ranges. 2 Climate of Southwestern Montana The climate of southwestern Montana is influenced by latitude, continentality, and altitudinal variation. The climate is characterized by low annual precipitation, large quantities of solar radiation, and wide daily and annual temperature fluctuations. The steppe habitat of this region (1400-1600 m altitude) is characterized by hot dry summers and cold winters with precipitation amounts increasing with higher elevations. Precipitation in the region typically ranges from 20 to 35 cm annually depending on the local elevation. The mountain ranges surrounding the Gallatin Valley of southwest Montana begin at elevations around 1800 m and rise to heights from 2800 to 3500 m. Mean annual precipitation in the mountains varies from 25 cm at lower elevations to 50 cm at higher elevations. Most of the precipitation comes in the form of snow. Tree line in the mountains begins around 2700 to 2800 m depending on the local conditions. Conditions, which create such variable climates, include elevation, slope angle, and aspect. For example, mountainsides facing southwest tend to be the warmest and driest slopes because earlier evaporation of dew allows the sun to more rapidly heat the soil surface; in contrast north-facing slopes tend to be wetter and cooler (Shreve 1924, Greg 1963). At any time, there can be great variation in weather conditions over short distances. 3 Vegetation of Southwest Montana Grasslands composed mainly of native vegetation were chosen for this study. Sampling sites were chosen from four regional or landscape vegetation categories: steppe, piedmont, montane, and alpine (Price, 1981). The piedmont or foothill zone generally represents an integration of steppe and montane vegetation, and is an area where precipitation tends to increase most abruptly. The steppe zone is found in the central portion of the Gallatin Valley, a sinking block between faults from which rise the Bridger and the Gallatin mountain ranges to the east and south, respectively. The elevation of the valley varies from 1200 m to 1600 m. In the western portion of the valley, grassland sites are relatively hot and dry with some vegetation characteristics similar to those found in the Great Basin desert (Mueggler and Stewart 1983). As the valley floor rises to the east into the foothills of the Bridger Mountains and Gallatin Range (1400 m to 1800 m), there is a corresponding increase in mean annual precipitation from 35 to 50 cm. The steppe/montane interface or foothill zone occurs at an elevation between 1500 m and 1700 m. The montane region is characterized by cool, moist coniferous forest. Montane grasslands occur at elevations between 1800 m to 2700 m with vegetation varying dramatically within this area due to local differences in conditions governed by slope angle, aspect, soil type, and elevation. The alpine regions in the Bridger and Gallatin ranges are found at elevations 4 between 2700m and 3100m. In this region, we see a shift from the coniferous forest to alpine tundra. The growing season is extremely short and plants possess adaptations to survive the harsh climate. These adaptations include a bunched and shortened morphology and increased plant hair to increase insulation. Many of these sites, including the montane and alpine sites, have been grazed by both domestic and wild animals, but their precise grazing history is unknown. During the course of the study, grazing was observed on the steppe sites, one foothills site, and one montane site. Grasshoppers in Steppe and Mountain Environments In mountainous regions, the effect of altitude on the evolution of insect morphology is similar to that of latitude. A number of insect traits vary with increasing altitude (Mani 1968, Alexander and Hilliard 1968, Somme 1982). First, the reduction in body size with increasing altitude is one of the most striking forms of variation within and between species (Mani 1968). Second, insects tend to exhibit increased aptery with increasing altitude. Uvarov (1977) suggested that wing reduction in alpine grasshoppers was an important adaptation to the alpine environment arising from the selection pressure of the climate. Uvarov felt that given the short growing season, it is more important to put more of the energy stores toward reproductive structures rather than wings. 5 Third, some insect species exhibit increased melanism with increased altitude. Kingsolver (1983, 1985b) documented the importance of melanism in pierine butterflies at high elevations and its relation to thermoregulation. Fourth, insects exhibit increased cold-hardiness with increasing elevation (e.g. Salt 1961, Duman et al. 1982, Somme 1982). Physiological cold-hardiness seems to come from one of two methods (e.g. Salt 1961, Duman et al. 1982, Somme 1982). Freezing-tolerant species are able to survive the formation of ice crystals in their tissue. In contrast, freezing-susceptible insects rely on supercooling to survive. In addition to these processes, insects inhabiting higher elevations can perform their daily activities at temperatures lower than those of their lowland counterparts (Gillis and Smeigh 1985). Lastly, due to cooler temperatures, the rate of development may be retarded at higher altitudes. This may result in a need for two or more years to complete the life cycle. It may also result in the compression of the length of each instar or a reduced number of instars. For example, White (1978) found that three species of grasshoppers in the Craigiebum Range of New Zealand had two-year life cycles. Distribution of Acrididae Approximately 200 species of Acrididae have been identified in the grasslands of the western United States, (Pfadt, 1968). Kemp et al. (1990a) 6 identified more than forty species inhabiting steppe and foothill regions in the Gallatin Valley of Montana. Unfortunately, the economic impact of most of these species is unknown, because they have not been studied in enough detail to understand their true status as pests. However, two grasshopper species frequently considered as pests of Montana agriculture include Melanoplus sanguinipes (Fabricius) and Aulocara ellioti Thomas. Alexander and Hilliard (1969), working in the Colorado Rocky Mountains, cataloged 94 species of Orthoptera, including 73 species of Acrididae along an altitudinal gradient from 1530 m to 4265 m. The gradient was divided into 1000 ft bands. Each band was placed in one of nine vegetation zones, based on vegetation patterns and dominant plant species. (The results showed a clear segregation of particular grasshopper species into distinct zones along the gradient and a reduction in species numbers with increasing elevation. There was also evidence that some species showed compressed life cycles during development from nymph to adult and an expanded duration of the egg phase. This study was descriptive in nature and did not quantitatively examine how factors other than vegetative zones influenced distribution or phenology. Scoggen and Brusven (1973), in a study to detect grasshopper species association with plant communities, used several biotic and abiotic factors in a cluster analysis. Their study included an altitudinal gradient of 2000 m. Their findings showed that, out of 10 plant communities, five vegetation communities 7 were significant indicators of certain assemblages of grasshopper species. However, their analysis was not based on quantitative data using grasshopper abundance. Rather, they used the arbitrary terms "accidental","occasional", or "common". Evans (1987) examined how fire, topography, and vegetation influenced grasshopper communities on tall grass prairie. The scale of study was defined and smaller than most previous work (Alexander and Hilliard 1968; Scoggen and Brusven 1973). Using detrended correspondence analysis (DCA), Evans showed that grasshopper distribution was most strongly related to the frequency of bum and the soil type. He suggested that this was probably due to the effect of these factors on the local plant communities. However, Evans noted that his study could not differentiate whether grasshopper assemblages were being influenced by plant species or by the physical structure of the environment. Work by Kemp et al. (1990a) revealed non-random distributions of grasshopper species existed along a rangeland environmental gradient. This gradient consisted of changes in elevation, moisture, and plant communities. The vegetation communities analyzed were chosen based on the concept of habitat type (Mueggler and Stewart 1980). These gradients appeared to play a role in structuring grasshopper community composition. Grasshopper community composition was found to vary both between habitat type patches and within habitat type patches. Significant associations were found between the 8 number of species as represented by subfamily within a given habitat type, suggesting that, given information on patch resources, one could generally predict species distribution. Yet it remains unknown how dynamic these associations are in space and time. Phenology of Acrididae Knowledge of life cycle variation has both applied and theoretical ramifications for ecologists and pest managers. Kemp (1991) discussed the value of understanding phenological patterns for pest control purposes. First, understanding how environmental gradients affect life history patterns can lead to more cost efficient and timely pest control. Second, studying life history variation, particularly within species, may illuminate factors that influence adaptation and speciation. Early studies of grasshopper development noted that low temperatures accelerated the development of eggs (e.g. Uvarov 1931, Moore 1945, Parker 1930, Bodine 1928). These low temperatures were below what was considered the lower threshold of development (sometimes below 0° c), but above a level that was injurious. Researchers from this period also noted that these thresholds seemed to vary with each life stage (Uvarov 1931). This phenomena suggested to Uvarov that the use of a mean daily temperature was inappropriate for analyzing questions regarding development. 9 Few studies have examined intraspecific variation in development at different altitudes. Handford (1961), found that mean inStars of Camnula pellucida (Seudder) for a given date were lower at higher elevation sites in the 900 foot elevation gradient study. Handford also calculated degree-days above 60° F from air temperatures recorded from a nearby airport. He chose 60° F because the lower threshold for Camnula pellucida (Scudder) was unknown at the time. Dingle et al. (1990) studied how the life cycle of M. sanguinipes varied with altitude in the Sierra Mountains of California. They sampled six populations from sites at coastal elevations of 90 m to mountain elevations of 2700 m. They computed degree-days from air and soil temperatures using weather stations on each site, with 15° C the lower developmental threshold temperature. Phenologies were calculated as percent instar composition by date. At higher altitudes, they found later spring emergence and a more rapid passage through the instars to maturity. They attributed these differences to the length of the growing seasons based on soil temperatures. They believe that air temperatures do not produce enough degree-days to complete nymphal development. If, however, soil temperatures are used there is adequate heat available for full grasshopper development to occur. Using laboratory experiments, Dingle’s study also showed all populations of M. sanguinipes to be univoltine, but populations varied in their intensity of 10 egg diapause and rates of growth. At high altitudes females laid diapausing eggs. At mid-altitude females laid a mixture diapausing and nondiapausing eggs. They believed these differences reflected genetic variation among populations. Objectives of distribution and phenology studies First, using the methods of Kemp et al. (1990a) as a model, I examined local variation within mountain ranges by sampling mountain slopes of different elevation and aspect to determine local grasshopper species variation. My null hypothesis was that there was no variation in species assemblages between sites of different elevation and vegetation. Second, I examined how grasshopper species varied from steppe elevations to the highest elevations of the surrounding mountain ranges. Third, I examined variation between the Bridger and Gallatin mountain ranges to see how species varied between mountains. The objectives of the development study were, first, to examine intraspecific variation in phenology of grasshopper species from sites of varying altitude and aspect. My null hypothesis was that there was no intraspecific variation in grasshopper phenology between patches. Second, I wanted to use the concept of degree-days to measure intraspecific and interspecific variation in phenology. Third, I wanted to examine interspecific variation in grasshopper phenology between species inhabiting local patches or having divergent ranges. 11 MATERIALS AND METHODS Study Sites The study area was located in the northern part of Gallatin County, Montana (longitudes l l l o40’- lll° 4 1 ’ east-west, latitudes 46 °00’-45 °30’north-south).. Sites were chosen from four regional vegetation zones (Price, 1981): steppe, montane, alpine, and the transition between steppe and montane often known as the foothill zone. At each site an area of 50 m x 80 m was laid out. Within this area, 9 linear transects were constructed using flags as markers. I chose two steppe sites from those sampled by Kemp et al. 1990a near Three Forks, Gallatin County, MT. Steppe sites were between 1400 m and 1500 m in elevation and were flat in their aspect. Two foothill sites located on the western front of the Bridger and Gallatin ranges were sampled. The first, located at the entrance of North Cottonwood Creek in the Bridger range at an elevation of 11550 m (longitude 111°- east-west, latitude TM0OT north-south). The second western foothill site was located at the entrance of Hyalite Canyon near the Gallatin range at an elevation of approximately 1850m (longitude I l l 0OI ’east-west, latitude 45°33’ north-south). A third site, located on the eastern slopes of the Bridgers, at approximately 1950 m in the Brackett Creek drainage was sampled (longitude 110°52’ east-west, latitude 45 °50’north-south). The montane and alpine sites were selected from the 12 Bridger Mountain Range and the Gallatin Mountain Range. In the Bridger range two sites were selected for weekly sampling. On the west side of the range at an elevation of 2270 m a west-facing meadow was chosen located between Corbly Gulch and Limestone Canyon (Longitude 111°00’ east-west, latitude 45°52’ north-south. On the east side of the range, located near Fairy Lake a mountain meadow that was relatively flat in aspect and approximately 2350 m in elevation was selected (Longitude 111°02’ east-west, latitude 45°55’ east-west). An east-facing montane site located in the Gallatin range was sampled. It is located in the Lick Creek drainage at an elevation of 2275 m (longitude 111 °0Least-west, latitude 45°33’ north-south). Two Bridger alpine sites were selected. The west-facing site is located on a western slope directly below Sacagawea Peak at an elevation of 2950 m (longitude 111°02’ east-west, latitude 45°53’ north-south). The east-facing Bridger alpine site is located on the north-east side of Hardscrabble Peak at approximately 2850 m (longitude I l l 0OV east-west, latitude 45°55’ north-south). In the Gallatin range, an east-facing alpine site was chosen on the slopes of Mt. Blackmore, at an elevation of 3050 m (longitude 111°00’,latitude 45°32’). Sampling Techniques Grasshoppers Sampling methodology consisted of two hundred sweeps each traversing 13 an arc of 180° with a 40 cm diameter sweep net, along permanent linear transects. These 200 sweeps were divided into 50 sweeps along four transects randomly chosen each sampling period. In 1991, the sampling period was between 0900 h and 1600 h. In 1992, the sampling period was shortened compressed to three hours from 1200 h to 1500 h. The period was shortened in an attempt to increase relative abundance, particularly in the mountains. Samples were collected from each site every 7-10 days. In 1991, the seasonal sampling period ran from 15 June to 25 August. In 1992, the sampling period ran from 5 May to 25 August. Samples were placed on ice until they could be delivered to a laboratory freezer. Plants Vegetation was sampled from mid-June through August of 1992. Twenty to thirty quadrants (20 cm x 50 cm) were sampled for cover (Daubenmire 1959) and species type along a transect within the area of the grasshopper sampling site. In addition to percent canopy coverage for each plant species, litter, and bare ground coverage were estimated. The variation in the number of quadrants sampled was due to traveling and weather constraints. Temperature Data and Phenology Temperature data was collected from stations located at six of the eleven sites (see Table I for specific sites). The foothill station was located on the 14 I western front of the Bridger range. The other stations that I installed consisted of two on the west side of the Bridgers and the two on the east side of the Gallatins located in the Hyalite region. Stations were erected in the central portion of each site. A Omnidata two channel Li-cor device was used to take hourly air (5cm) and subsurface (-5mm) temperatures. Temperature data was reduced to daily maximums and minimums. From this data, degree days were calculated from 5 May to dates on which only adults were collected in samples. Degree days were calculated only for sanguinipes and Mi oregonensis. This choice was due to their abundance across sites and thermoregulatory studies, that I carried out. The base temperature chosen for calculating degree days for M=. sanguinipes was 17 0C (Kemp and Dennis 1991). The base temperature chosen for Melanoplus oregonensis Thomas was 5 0C. There is no available research data on M. oregonensis developmental thresholds. I chose 5°C for two reasons, first, from thermoregulatory studies of Moregonensis. I found that alpine populations were able to walk and jump at temperatures in this range. Second, I found nymphs in the alpine regions at temperatures below 10°C. Degree days were calculated beginning with the installation date of the temperature recording devices. For the 1991 field season, installation occurred after hatch particularly at the lower elevation sites. In 1992, all stations were installed prior to hatch (i.e. approximately Julian date 125) (Kemp and Dennis 15 1991). Four species were chosen for phonological analysis. These choices reflected three factors: I) their collection from early nymph stages through adulthood across two or more vegetation zones or at sites of different aspect, 2) their occurrence in numbers high enough to analyze, and 3) my ability to make accurate identifications of nymphs. Identification of Acrididae Most of the Acrididae found from these sites are well-documented species, particularly those in the subfamilies Gomphocerinae, Acridinae, and Oedipodinae (Otte 1981). Adults from the steppe and foothill regions were identified using keys from Pfadt (1989), Capinera and Sechrist (1982), Hebard (1928), and Brooks (1958). Nymphs from these regions were identified using Brusven (1972), Scoggan and Brusven (1972), and Hebard (1928). Mountain species, except for certain species of Melanoplus adults and nymphs, could often be identified using the above keys. The subfamily Melanoplinae is a poorly understood group taxonomically (Otte 1993). Few taxonomic keys even refer to certain alpine Melanoplus. in particular M. oregonensis and Melanoplus montanus (Thomas). To aid in the identification of these, Brooks(1913) and Alexander (1948) were used extensively. Information on the nymphs of these problematic species is even more difficult to find. Typically, adult male 16 characteristics, are well enough developed by the fourth instar to allow identification. Identification of the early instars was possible by comparing them with later instars and adults and male morphology was distinguishable often by the fourth instar. Data Analysis Given that species along the gradient dropped out of samples with changes in elevation and aspect, I used detrended correspondence analysis (DCA) (ter Braak 1988). DCA is an ordination technique that can summarize count data and arrange it into two-dimensional space. Within these dimensions most of the variation among sites will be accounted for. Increasing distance between samples along the axis shows a decrease in similarity. The axis scores were correlated to site variables using Spearmann rank correlation (rs). 17 RESULTS Species Distribution Grasshoppers I collected twenty-five species of Acrididae from the eleven sites during two field seasons (Table I). The number of species tended to decrease with increasing elevation (rs =-0.72, P = 0.012)(Fig. I). The total number of individuals collected also decreased with increasing altitude (rs =-0.68,P =0.021, Fig. 2). The montane elevation sites (2250 - 2350 m), showed a slight increase in species numbers and individuals, before decreasing again at the alpine elevation sites. I found an uneven distribution of grasshopper subfamilies along the environmental gradient (Table I). The most obvious trend was the increase in the percent of Melanoplinae (all Melanoplus spp.) with an increase in altitude: steppe 31%, foothills 56%, montane 64%, and alpine 88% (chi-square 2x4 contingency table analysis, x2 = 6.99, P = 0.07). In fact, only I of 102 grasshoppers collected on the alpine sites was not a Melanoplus. Melanoplus comprised a minority of species only on the steppe sites. However, even here they made up the majority of grasshoppers collected, because of the great abundance of Melanoplus infantilis Scudder and M. sanpuinipes (74% at the 1400 m site and 69% at the 1500 m site). Other species Table I. Grasshopper (Orthoptera: Acrididae) Species Collected. Number of Individuals Collected by Elevation(meters) and Aspect. Superscript key: I - Only nymphs collected, 2 - Only adults collected, 3 - Both nymphs and adults collected. AsterisksQ indicates sites with temperature recording stations. Alpine Montane Foothills Steppe 2850 2950* 3050* 2350* 1950 2250* 2275* 1400* 1500* 1800*. 1850 W NE E SW E SE SW E W Flat Flat Oedipodlnae Arphia pseudoneatoma1 Arphia conspersa2 Camnula pellucida2 Spharagemon collare1 Trachyrhachys kiowa3 T r i m e r o t r o p i s suffusa' Xanthippus corallipes3 Subtotal - — - — — I 2 2 I 4 2 19 30 — - - — — - — — — - — — 7 — 2 4 z. 2 3 6 — — — - 24 >7 A ? Gomphocerinae & Acridinae Aeropedellus clavatus3 Ageneotettix deorum3 Amphitornus coloradus3 Aulocara eliotti3 Chorthippus curtipennis3 Psoleossa delicatula3 Subtotal Helanoplinae Helanoplus alpinus2 Helanoplus bruneri3 Helanoplus bivittatus3 Helanoplus confusus2 Helanoplus dawsonii3 Helanoplus fasciatus3 Helanoplus huroni2 Helanoplus infantalis3 Helanoplus montanus2 Helanoplus oregonensis3 Helanoplus packardii3 Helanoplus sanguinipes3 Subtotal 11 98 20 111 5 — 5 25 54 44 48 2 -240 I 38 82 649 770 14 185 _ - — 260 46 168 474 - 15 92 7 90 — - 12 — i 50 266 — — - — — — — - - - - 5 — — I 5 - — — 9 9 I 5 — — — - — — — — 12- W „ _ . — — - — - — — — — — — — — — 2 2 17 25 70 103 — — — 17 25 — 70 103 - - - 3 — - - 21 — I I I “ I — — 2 5 — — — — — — — — — — 20 - 9 3 14 12 — 7 24 11 — — 42 13 11 — — - — — — 5 8 2 75 I 44 - 89 — 88 25 — — — 3 — — 188 .I 218 — 20 9 6 ? I 87 2 22 91 I S 26 * 12 11 cn 10 CD U CD 9 Ql if) O 8 7 6 CD 5 E 4 Z5 Z 3 2 __ I 1000 1500 2000 2500 Elevation (m ) Figure I. Num be r of S p e c i e s a s a function of elevation. 3000 3500 \ C/) O 3 "O > 't5 C ~ I 100 1050 1000 950 900 850 800 750 700 650 600 r - - « ISS: S HS : c ^ z 350 300 250 200 - 150 100 50 - 13 14 15 16 17 18 O O 19 20 21 22 23 24 25 26 Elevation x 100 (m ) Figure 2. Number of Individuals collected from each sampling site as represented by elevation. 27 28 29 30 31 21 replaced these two as the most abundant Melanoplus in other zones. Melanoplus bivitattus (Say) (35%) and Melanoplus dawsonii (Scudder) (28%) were most abundant in the middle of the gradient (foothills and montane). Melanoplus oregonensis predominated at highest altitudes (montane and alpine), where it made up 91% of the grasshoppers collected. The fact that nymphs of Melanoplus alpinus Scudder, Melanoplus bruneri Scudder, M. bivittatus. and M. oregonensis were collected on the montane sites suggests that adults of these species oviposit in this zone. Only M. oregonensis was collected throughout its life cycle in the alpine zone. The greatest diversity of non-melanoplines also occurred on the steppe sites. I found adults of only one gomphocerine on the foothill and montane sites. Although low in abundance at all sites, Camnula pellucida was the only oedipodine collected in all four zones. In contrast, others occurred only on the steppe sites and Trimerotropis suffusa was collected only in the montane zone. No species of Gomphoceiinae were widely distributed across the gradient. The most commonly collected species, Chorthippus curtipennis (Harris) was found at five sites in two zones. Comparisons between the two mountain ranges showed montane species numbers ranging between 6 and 10. The west-facing montane site in the Bridgers (2250 m) contained seven species. The east-facing montane Bridger sites contained six species, while the east-facing montane Gallatin site produced 22 10 species. The Bridger alpine site (east-facing) contained only one species (M. oregonensis), while the east-facing Gallatin alpine site yielded four species. Melanoplus oregonensis also predominated at this site. Table 2 shows the first collection dates of second instars (1992 data only) from sites of different aspects. Chorthippus curtipennis. found predominantly in the montane zone, was collected 9 days earlier (ID 181) at the east-facing Gallatin site and the west-facing Bridger site than the east-facing Bridger site (JD190). This Bridger site is approximately 100 meters higher than the other 2 sites. A similar situation is seen for M. bivittatus in the montane zone. All collection dates were within a four day range. M. oregonensis showed a greater range of variation within vegetation zones. At montane elevations, the Bridget west-facing site was the earliest (ID 145) while the Gallatin east-facing site was the latest (JD158). In the alpine region, the Gallatin east-facing site was the earliest (JD180), while the Bridget east-facing site was much later (JD210). The Bridger east-facing site had large amounts of snow until early July. Grasshopper DCA Results The DCA on grasshopper study sites, showed that a high proportion of variation in the samples is accounted for by the first DCA axis (Fig. 3). Ninety-four percent of the variance in species data was accounted for in axis I. Spearmann rank correlations showed that axis I scores were significantly 23 Table 2. Julian Dates for the first collection of 2nd instars from sites o f varying aspect within a given vegetation zone. These dates are for 1992 data only. Dates with (G) indicate a Gallatin Range site, all others are Bridger Range sites. Vegetation Zone C. c u r t i p e n n i s M . bivittatus M. o r e g o n e n s i s Foothill East West Montane East West 160 152 East Alpine West 190 181(G ) 18 1 166 1 6 9 (G) 168 150 1 5 8 (G) 145 210 1 8 0 (G) 2.5 M%Sbm 2.0 2850 m 1.5 Axis C\2 1600 m 2275 m 2250 m 1500 m 1.0 1800 m 0.5 2 1850 m 1950 0.0 —0.5 -------------- '---------------- L -I 0 1 12350 m j_________ i__________ i__________ i__________ i 2 3 4 5 6 Axis I Fig. 3. D e n t r e n d e d C o r r e s p o n d e n c e Analysis of 11 g r a s s h o p p e r sa m p li n g s i t e s a c r o s s 4 v e g e t a t i o n zones. a n d 0.19, r e s p e c t i v e l y . E ig e n v a lu e s for x a n d y axes a r e 0.94 25 correlated with elevation, precipitation, proportion of grasses and forbs, and total number of plant species (Table 3). Axis 2 accounted for nineteen percent of the variance in the data (Fig. 3). Axis 2 correlations were significantly correlated (P<0.05) with elevation, precipitation, and total number of plant species. Figure 4 shows the DCA site scores for the grasshopper species. The species are distributed along the plot in a manner similar to the study sites. Species that tend to be found in steppe regions are found toward the left portion , of the plot, while foothill, montane, and alpine species are increasingly to the right of the plot. Plant Distribution Table 4 lists the plant species used for DCA including their percent canopy coverage by site. My studies of steppe vegetation showed that these grassland sites were dominated by Stipa comata Trin. & Rupr. and Bouteloua gracilis (H.B.K.) Lag. exSteud. These plant communities occur in the most arid of the study sites, with annual precipitation of 20-35 cm. Other grasses such as Agropyron spicatum (Pursh) Scribn., Agropyron smithii Rydb., Carex filifolia Nutt., Koeleria cristata (L.) Pers., and Poa sanbergii Vasey were found in lesser densities. Forb species associated with this grassland type include; Astragalus spp.. Aster spp.. Liatris punctata (Hook.), Phlox hoodii Richards., and 26 T a b le 3 . C o r r e l a t i o n s co m p u te d f o r c o m p a r is o n s b e tw e e n DCA a x e s s c o r e s and e n v ir o n m e n t a l v a r i a b l e s C o r r e la tio n s r/ P DCA: G r a s s h o p p e r C o m m u n itie s A x is I s c o r e s A x is 2 s c o r s e DCA: v s . e le v a tio n v s . p r e c ip ita tio n v s. asp ect v s . p r o p o r tio n o f g ra sses v s . p r o p o r tio n o f fo r b s v s . T o ta l # o f p la n t s p e c ie s 0.82 0.88 0.70 -0.76 0.002 0.000 0.163 0.006 -0.73 0.002 0.61 0.001 v s . e le v a tio n v s . p r e c ip ita tio n v s . asp ect v s . T o ta l # o f p la n t s p e c ie s 0.62 0.63 0.26 -0.47 0.038 0.035 0.432 0.021 v s . e le v a tio n v s . p r e c ip ita tio n vs. asp ect v s . p r o p o r tio n o f g ra sses v s . p r o p o r tio n o f fo r b s vs. to ta l # of p la n t s p e c ie s 0.01 0.01 0.25 -0.39 0.987 0.973 0.480 0.265 -0.37 0.289 0.62 0.060 v s . e le v a tio n v s . p r e c ip ita tio n vs. asp ect vs. to ta l # of p la n t s p e c ie s 0.73 0.71 0.67 -0.18 0.016 0.022 0.031 0.618 P l a n t C o m m u n itie s - A x is I s c o r e s A x is 2 s c o r e s 4 mm 2 mo me oec CV I mp CA) X igs ms op mdmbi mb O CC -I mf ts -2 I -3 -2 -I J___________ I 0 I 2 3 _L 4 to mh J __ 5 j 6 Axis I Fig. 4. Detrended C orrespondence Analysis of 25 grasshopper sp ecies for 11 sam pling sites across 4 vegetation zones. Eigenvalues for x and y are 0.94 and 0.19, respectively. Key to grasshopper species: K C - A r p h ia c o n s p e r s a , A D - A g e n e o te ttix d e o r u m , A e C - A e r o p e d e llu s c la v a tu s , A m C - A m p h ito m u s c o lo r a d u s , A P - A r p h ia p s e u d o n e to m e , C C -C h o r th ip p u s c u r tip e n n is , C P C a m n u la p e llu c id a , M A -M e la n o p L u s a lp in u s , M B -M e la n o p lu s b r u n e r i, M B i-M e la n o p lu s b iv itta tu s , M C -M e L a n o p lu s c o n f u s u s , M D -M e L a n o p lu s d a w s o n ii, MF—M e la n o p lu s f a s c i a t u s , M H - M e la n o p lu s h u ro n i, M m - M e la n o p lu s m o n ta n u s , M o -M e la n o p lu s o r e g o n e n is is , M p - M e la n o p lu s p a c k a r d ii, M s M e la n o p lu s s a n g u in ip e s , P d - P s o l e o s s a d e lic u tu la , S c - S p h a r a g e m o n c o lla r e , T k - T r a c h y r h a c y s k io w a , T s - T r i m e r o t r o p i s s u f f u s a , X c - X a n t h i p p u s c o r a llip e s . Table 4. Plant species used for DCA, represented by percentage of canopy coverage. S te p p e 1400 1500 F la t F la t F o o th ills 1800 1850 W SW 2250 SW M o n ta n e 2275 E 2350 SE 2850 NE — — — — — — — — — — ii - 8 — — 11 4 — 3 5 2 6 I 5 G rasses Stlpa comata Bouteloua gracilis Agropyron splcatum Agropyron smithii Carex filifolia Koeleria cristata Poa sanbergii Poa pratensis Festuca idahoensis P o a spp. Phleum alpinum Danthonia intermedia C a r e x spp. Deschampsia caespitosa 21 12 4 I 5 4 — - 21 6 18 — 19 10 I — — — - 4 13 3 — I I 4 29 3 5 2 I 2 — 3 I 7 26 I 4 I 2 I — 2 — 16 I 2 4 — 10 3 2 2 — 5 7 A lp in e 2950 W 3050 E — — — — — — 14 3 5 2 11 15 11 4 3 I — 5 13 22 to OO Forbs A s t r a g a l u s spp. A s t e r spp. Liatris punctata Lupinus sericeus Phlox hoodii Achillea millefolium Artemisia ludoviciana Balsamorhiza sagitatta Guara coccinea Geranium viscosissimum Silene acaulis Gentian parryi Potentilla gracilis T r i f o l i u m spp. Helianthus rigidus 7 5 5 3 4 I 7 3 — — — — 11 — - — — 10 20 12 6 8 I 32 I 21 8 8 21 I I 3 8 I 3 2 15 2 8 I 3 5 10 12 12 2 13 2 - - 8 12 7 - - 4 3 14 — 2 2 19 3 16 — — — 16 — I7 — — — — — - 10 I — — — — - I 13 5 — 17 3 3 — — — — - 2 3 — — I 2 - — — — 5 2 7 6 — 7 I 4 2 — 29 Sphaeralcea coccinea (Pursh) Rydb. In the foothill zone, the dominant grasses were Festuca idahoensis (Elmer.) and Agropyron spicatum Rydb. (Mueggler and Stewart 1974). Other important grasses in this community include Danthonia intermedia (Vasey), Koeleria cristata (L.) Pers., Poa pratensis L., and Stipa comata. There is also a greater representation of forbs, the dominant species being Achillea millefolium L., Artemisia ludoviciana Nutt., Balsamorhiza sagitatta (Pursh) Nutt., Guara coccinea Pursh., Liatris punctata Hook., and Lupinus sericeus Kell. The vegetation found at the montane sites included Festuca idahoensis in association with the grasses Ai spicatum. and Deschampsia caespitosa (L.) Beauv, and the forbs Achillea millefolium. Lupinus sericeus Kell., Geranium viscosissimum F. & M., Danthonia spp., and Poa spp. Alpine vegetation typically consisted of Festuca idahoensis in association with D .caespitosa. Silene acaulis L., and Gentian parryi. Other grass species present included Phleum alpinum L., and Carex spp. Forbs included Potentilla diversifola L. and Trifolium spp. Plant DCA Results Plant species scores, using DCA, for axis I accounted for seventy-three percent of the data variance (Figure 5). Spearmann rank correlations showed that axis I scores were not significantly correlated to any environmental 3 .0 r2350 m 2.5 2 .0 C\2 Axis W < X C 1.5 2850 m • r— 1500 m 2250 m 1 .0 2275 m 1600 m . 1850 m 1950 m g 0.5 0 .0 — 1800 m l -0 .5 1 0 l l 1 l 2 l 3 l 4 l 5 6 Axis I Fig. 5. D e t r e n d e d C o r r e s p o n d e n c e Analysis of 11 p l a n t c o m m u n i t i e s a c r o s s 4 v e g e t a t i o n z o n es. 0.44, r e s p e c t iv e ly . E ig e n v a lu e s for x a n d y axes a r e 0.73 a n d 31 variables (P<0.05) (Table 3). Axis 2 scores were significantly correlated with elevation, precipitation, and aspect. Grasshopper Phenology I chose C. curtipennis. M. sanguinipes. M. bivittatus. and M. oregonensis for phonological analysis. Their selection was based on three criteria. First, their distribution needed was broad enough to allow for comparisons between vegetation zones or multiple sites within a vegetation zone. Second, they were abundant enough to study. Third, I was able to identify the species, particularly when studying early instars. The determination that a species completed its development at a site was based on the collection of that species throughout its instar development period and as an adult. The seasonal phenologies were computed from weekly sweep net samples and the mean instar was calculated for a given collection date. It is not known if these populations are univoltine. Melanoplus sanguinipes Melanoplus sanguinipes was found to complete its development at the two steppe sites and one of the foothill zone sites located on the western side of the Bridger range (Figure 6). In 1991, this grasshopper was only collected from the two steppe sites. I found no apparent differences in development between the two rangeland sites in either year. In 1992, M. sanguinipes was collected from the 1850 m foothill site as well. Nymphs appeared at the foothill site 7 1991 MEAN INSTAR 6 O 5 4 3 O = I 400m □ = 1500m 2 O I I 225 0 150 200 175 1992 MEAN INSTAR w to O =1 400m JULIAN F ig u re 6. □ = I 500m A = I 800m DATE P l o t s of M. s a n g u in ip e s d e v e l o p m e n t as m e a n i n s t a r a g a i n s t Julian d a te . 33 approximately 15 days later than the rangeland sites, but adults appeared approximately on the same dates for all sites. Spring emergence was approximately 10 days earlier in 1992 for all sites. Adult M- sanguinpes not only appeared later in 1991 than 1992 (Fig. 6), but required a greater number of degree days to complete development (Fig. 7). This may reflect the colder and wetter spring of 1991. Comparisons between steppe and foothill sites for 1992 only (no nymphs of M- sanguinipes were collected from the foothill site in 1991) show that M- sanguinipes at the foothill site, completed development in fewer accumulated degree-days then on the steppe sites. Melanoplus bivittatus M- bivittatus was found throughout its life cycle at all foothill and montane sites. Figure 8 shows development for all sites. In 1991, M- bivittatus was only collected in sweep net samples from one Bridger Mountains site, although I observed the grasshopper at all Bridger mountain sites collected in 1991. In the Bridger mountains in 1992, M- bivittatus was first collected at 1800 m on Julian date 143. At the elevations 2250m and 2350 m, it was first collected on date 157. This time lag between elevations was observed throughout its development too. A similar initial time lag was observed in the Gallatin Mountains between sites 1850 m and 2275 m, but the differences disappeared later in the season. □ 6 I INSTAR □ O V □ "1> ii ^ □ □ MEAN -r<> v • = Steppe (1991) v = Steppe (1992) □ = FoothiIIsO 992) i_____________I_____________I_____________I_____________I____________ I____________ I____________ l _ 0 100 200 300 400 500 600 700 _I 800 ACCUMULATED DEGREE DAYS Fig. 7. Mean i n s t a r of M. s a n g u in ip e s p l o t t e d a g a i n s t a c c u m u l a t e d d e g r e e - d a y s ( b a s e 17°C) fo r two p o p u l a t i o n s o v e r two field seaso n s. Gallatin M o u n ta in s B r i d g e r M o u n t a in s 7 1800m V = 2275m 1991 In s ta r 5 Mean 6 3 4 V 2 1 I - 0 0 - 7 INSTAR 5 MEAN 6 3 O — 1800m V □ = 2250m = 2350m j x 150 O V = 175 200 225 250 1850m w Ul = 2275m 4 2 I 0 Julian Da t e Julian Date F i g u r e 8. P l o t s of M. b i v i t ta t u s d e v e l o p m e n t p r e s e n t e d as m e a n i n s t a r as a as a f u n c t i o n of Ju lian d a te . 36 Chorthippus curtipennis C. curtipennis was collected from all montane sites sampled in both mountain ranges. In 1991, only two montane sites were sampled (Fig. 9), the sites were similar in elevation, but the Bridger site was west-facing and the Gallatin site was east-facing. Nymphal development was similar at the two sites throughout the season with adults appearing between dates 220 and 230. In 1992, a lower montane site was sampled in the Bridger range (1950 m). This site when compared to the 2250 m site shows that nymph development began earlier and instars progressed faster than the higher site. However, adults from both sites appeared at the same time. Comparisons of development between years shows that nymphs appeared much earlier in 1992 than in 1991. Melanoplus oregonensis M- oregonensis completed its life cycle at all six montane or alpine sites. In 1991, the difference between montane and alpine hatch dates was approximately one week in both the Bridger and Gallatin mountainss (Fig. 10). In 1992, there were three to five week differences between the montane and the alpine hatch. There were no differences in the duration of instars between montane and alpine elevations. In the Bridgers, M- oregonensis on the montane sites appeared to reach G alla tin M ountain s Bridger M ountains L, CO -t-J W 6 5 a 4 C cd 3 CU V 2275 m = 2250 m Y 2 V 1 0 __ I___________ | 150 175 200 1950 m 2250 m 2350 m 225 250 = 2275 m 150 Julian Date 1992 175 200 Julian 225 250 Date F i g u r e 9. P l o t s of C h o r th ip p u s c u r tip e n n is d e v e l o p m e n t p r e s e n t e d a s m e a n i n s t a r as a f u n c t i o n of Julian d a t e . Gallatin Mountains Bridger Mountains 7 7 Mean Instar 6 5 4 3 3 ^ 2 V 2 O =2250 m V =2950 m "V I __I___________ I 0 150 175 O = 2275 m V = 30 5 0 m I ---- 1--------- 1 0 225 200 250 Mean Instar O i ^ J u l i a n Date O □ V A =2250 =2350 =2930 =2850 m m m m T V V O = 2275 m V = 3050 m J u lia n Date F i g u r e 10. D e v e l o p m e n t o f M. o r e g o n e n s i s p r e s e n t e d a s m e a n i n s t a r byJu lian date. 39 equivalent stages of development with fewer accumulated degree-days than on the alpine sites. (Fig. 11). However, the alpine populations reached the adult stage about the same date as the montane sites in 1991, at both sites, development took longer than in 1992. This may reflect the colder and wetter spring of 1991. Gallatin Mountains Mean I n s t a r Bridger Mountains O =2250 m V = 2950 ci 800 1000 O =2275 m V =3050 m 1000 1200 Mean I n s t a r V V 1992 200 400 600 800 O =2250 m O =2275 m V = 2930 m V =3050 m 1000 1200 1400 A c c u m u l a t e d Degree Days 200 400 600 800 I 0001 2001 4001 6001 800 A c c u m u l a t e d Degr ee Days F ig u re 11. D e v e l o p m e n t of M. o r e g o n e n s is p r e s e n t e d as m e a n i n s t a r by a c c u m u l a t e d d e g r e e —days. 41 DISCUSSION Summary of Patterns of Grasshopper and Plant Distribution My surveys revealed variation in grasshopper community composition across four vegetation/elevation zones. DCA analysis on the grasshopper species showed different species and subfamily representation within each vegetation zone. Clustering of species occurred according to their representation in a given vegetation zone. Several clusters are apparent: I) species found only at the steppe sites including the Oedipodines Spharagemon collare (Scudder), Trachyrhachys Mowa Thomas, Xanthippus corallipes Haldeman, and the Gomphocerines Ageneotettix deorum (Scudder), Amphitomus coloradus (Thomas), A. ellioti Thomas, Psoloessa delicutula Scudder, and the Melanoplines Melanoplus infantalis Scudder and Melanoplus packardii Scudder 2) steppe/foothill species including Aeropedellus clavatus (Thomas) and M. sanguinipes 3) foothill/montane species including C. curtipennis. M. bruneri. M. bivittatus. and M. dawsonii 4) montane species including T. suffusa, M. alpinus Scudder, Melanoplus fasciatus (F. Walker), and Melanoplus huronii Blatchley 4) montane/alpine species including M. montanus and M. oregonenis. Lastly, both M. oregonensis and C. pellucida Scudder ranged from the foothills to the alpine region. C. pellucida was collected in small numbers (5 or less) from several sites within the three zones, while M. oregonensis was 42 collected in relatively large numbers from montane and alpine sites whereas in the foothill region smaller numbers of individuals were collected only as adults. These clusters support the results shown in Table I of increasing percentage of melanoplinae presence with elevation. The greatest diversity of nonmelanoplinaes occurred in the steppe zone and C. curtipennis and M. bivitattus were restricted to the upper foothill and montane zone. The DCA on study sites showed non-random distribution patterns. For each vegetation zone, except the montane, study sites clustered according to their elevation, precipitation, and the number of plant species. Within zones, in spite of variation in the plant communities, I observed similar species at each study site. Study sites within both mountain ranges showed more similarities than differences. In the montane zone, there was an increase in plant richness relative to other zones, a comparable number of species relative to the lower zones, and population sizes that were comparable to lower vegetation zones. In the alpine region, there seemed to be decreases in plant richness, species numbers, and number of individuals. Comparisons between the two mountain ranges failed to show distinct differences. Environmental Correlates of Grasshopper Distribution Grasshopper communities can be evaluated on many spatial scales. This 43 study has shown that the elevation, precipitation, and changes in plant species at a landscape scale are correlated to changes in grasshopper communities. Several studies have found positive correlations between vegetation patterns and grasshopper communities (Scoggen and Brusven 1973, Otte 1976, Evans 1987, Kemp 1990a). These studies are providing indirect evidence for the driving force behind community structure. Joem (1986) summarized possible ecological mechanisms responsible for grasshopper community structure. These mechanisms included interspecific competition, species-specific host plant use, and species-specific microhabitat use. Kemp (1992) discusses possible facultative associations of grasshopper species based on available resources. Grasshopper feeding patterns can help explain the patterns I observed along this environmental gradient, regardless of how ephemeral they are. Melanoplines, which are primarily forb-feeders with a wide diet breath, showed an increasing presence with elevation. This coincides with an increase in forbs and species richness from the steppe region to the montane. Lawton et al. (1987) suggested that species richness declines with elevation due to diminishing resources and increasingly unfavorable environments. In reality, this picture is more complex than Lawton portrays. It is true that the growing season shortens at higher elevations. However, as seen in my study, precipitation and plant species richness often increase at montane elevations (Price 1981). The most likely factors limiting habitat use are the 44 available heat energy, the genetics, and the thermoregulatory abilities of a species. Montane sites supported larger population sizes than did the foothill sites. There also exists the occurrence of a slight mid-elevational rise in species numbers in the montane zone due to the presence of C. curtipennis and M. oregonensis (Table I.). There has been debate over mid-elevational peaks in species richness (Janzen 1973, Lawton et al. 1987, McCoy 1990). When comparing montane versus foothill sites (Table I), there is an increase in number of species and in population sizes. This suggests an increase in habitat complexity allowing for more species and larger population sizes. Furthermore, I collected the following species in montane habitats, including C. pellucida. A. clavatus. Melanoplus confusus (Scudder), Melanoplus fasciatus (F. Walker), Melanoplus dodgei (Thomas), and M. montanus as nymphs from montane areas not included in this study. This suggests that species numbers do not necessarily decrease with elevation. Montane communities of grasshoppers vary in species numbers and population size between patches. McCoy (1990) suggested that latitude, sampling regime, and disturbance produce different patterns of species richness along an elevational gradient. McCoy claims that short-term sampling produces mid-elevational peaks due to a more rapid species turnover rate at lower elevations. While he does not define in precise terms the differences between short-term and long-term, it is apparent from his work that short-term sampling is not continuous throughout the growing 45 season. I believe that my sampling would not be considered short-term within each growing season and, therefore, I would not be missing species due to turnover. Given the number of seasons sampled, I might be missing species shifts due to bi-voltinism or transitions in and out of a given patch in different years. Another factor which merits discussion is sampling technique. For example, given the low height of alpine vegetation, sweep net sampling may not be the best method for assessing species types and numbers. Anecdotically, my alpine sweep net counts never seemed to correspond to the number of grasshoppers I observed. McCoy’s reference to human disturbance may have some relevance to grasshopper populations in southwest Montana. Mid-elevation peaks could be caused by lower elevations being disturbed through agricultural practices and development. This would be fairly easy to test given the lack of reduction I observed in plant species with increasing elevation. If plant species stayed constant across sites of different elevation, then one could test for other effects such as disturbance. If disturbance does play a role in shifting species richness it could explain why we see certain species such as M. bivittatus inhabiting cropland borders and weedy areas yet find no significant populations of these species in steppe patches. Disturbance might also explain the narrow bands of distribution of certain species (e.g. M. dawsonii and C. curtipennis). 46 Alexander and Hilliard (1968) did not find mid-elevational peaks in their study of grasshopper distributions in the Colorado Rockies. Their data showed a steady decline in species number which they attributed to a shortening of the growing season with increasing altitude. A noticeable difference between our studies was their findings of much wider distributions for several species along the gradient. Based on their work, I expected to find certain species to occur across the entire gradient (e.g. A. clavatus. X. corallipes. and Mi sanguinipes). While M. sanguinipes and C. pellucida were collected across all zones, they were only found as adults above the foothill zone. In fact, I found no single species residing across the gradient. Species that occur in Colorado as high mountain species such as M. dawsonii were found in the foothill zone and into the lower montane zone. Melanoplus sanguinipes which from Alexander and Hilliard’s work occurred across their gradient as a resident species was relatively restricted when considering the regional scale. At smaller scales, certain species occurred in large numbers at several sites within certain zones (e.g. M. sanguinipes. M. oregonensis. and C. curtipennis). Species that were wide-ranging in Colorado, were found to be more restricted in their distribution. These differences suggest that regional environmental factors between the southern Rockies and the Northern Rockies tend to lower and narrow the distribution of certain grasshopper species. 47 Summary of Patterns of Grasshopper Development The four species chosen for the development study: M. sanguinipes. M. bivittatus. C. curtipennis. and M. oregonensis were concentrated in different vegetation zones along the environmental gradient. M. sanguinipes primarily inhabited the steppe study sites. Nymphs from the steppe sites began to emerge in May and develop over the course of the summer, with adults appearing from mid-July on. There was no strong divergence between the two steppe sites. In 1992, M. sanguinipes was collected from the 1850 m foothill site. Development at this site was compressed compared to the steppe sites with adults appearing at approximately the same dates for all sites (Fig. 6). Melanoplus bivittatus. an inhabitant of foothill and montane sites, showed some divergence in development, particularly in the Bridger mountains (Fig. 8). Nymphs followed a pattern of later emergence dates with increasing elevation. Foothill nymphs appeared first, then lower montane nymphs and finally upper montane nymphs. This pattern continued for their entire development cycle. Chorthippus curtipennis appears to be primarily a montane species. In 1991, nymphs appeared later in the growing season (Fig. 9). In 1992, nymphs appeared up to two weeks earlier yet finished development at approximately the same time as the 1991 populations. Chorthippus curtipennis was collected at multiple sites only in the Bridger mountains during 1992. Nymphs appeared earliest at the 1950 m site. Montane nymphs were collected approximately I 48 week later. The appearance of adults was not significantly later in time at higher elevations. Melanoplus oregonensis was collected primarily from montane and alpine sites. Again as with previous species, nymphs first collection date is later with increasing elevation. Using 1992 to illustrate differences in development, there was strong divergence of first collection dates between the montane and alpine sites, particularly in the Bridger mountains. This variance decreased as the populations moved to adulthood suggesting that the alpine populations were moving through their development more quickly. Bridger populations utilized equivalent amounts of heat for both field seasons (Fig. 11). In the Gallatin mountains, the patterns are not as clear. In 1991, due to missing most of the first 2 instars there is not a complete picture for the 2275 m site. The alpine population on Mt. Blackmore used nearly 600 degree-days from instar 3.5 to adulthood in 1991. In 1992, the Gallatin montane population used 1500 degree-days to complete their development. The alpine population again used nearly 600 degree-days for their entire development. This coincides with the length of winter conditions that persisted on Mt. Blackmore well into June of 1992. Environmental Correlates of Grasshopper Development The phenologies of the 4 species studied reflect differences in local 49 ambient conditions and length of the growing season. Populations appeared to be univoltine, but showed differences in the rate of development. All species demonstrated later development times and a compression of life cycles with increasing elevation. For three species, M. bivittatus. C. curtipennis. and M. oregonensis. slope aspect most likely affected development by influencing local temperature regimes and vegetation patterns. Most notably was the east-facing Bridger mountains alpine site where M. oregonensis was two weeks behind the , other two alpine sites (Table 3). Species appear to be more restricted than the available habitat and temperature regimes suggest. DCA scores did not correlate with air or ground temperatures (Table 2). This is a surprising result since we often consider temperatures to be a gauge of the heat energy in a patch and thus a major influence on life in the patch. From my temperature data, mountain air temperatures provide more available heat than ground temperatures during the spring and early summer. This is the opposite of the lower elevation sites where ground temperatures track air temperatures more evenly throughout the growing season. As long as snow is covering the ground it would seem that ground level heat is of little value to nymphs. Temperature profiles from sites in each zone show growing seasons start later with increasing elevation, air temperatures are similar to lower elevation sites and can be greater at montane elevations in July and August, and ground temperatures reflect the snow melt patterns but catch up 50 to lower elevation sites. Dingle et al (1990), believed that soil temperatures predict growing season length for M. sanguinipes along an altitudinal gradient. More research is needed to explore this question. Having seen first instar nymphs at alpine sites covered with large patches of snow and wet soil, I question whether nymphs are using the substrate as their primary source of heat. If hatching occurs before soils warm, nymphs may be using other microhabitats that provide warmer air temperatures such as exposed rocks. The phenologies for the melanoplines, to some extent, illustrate the community organization at local patches. Joem (1986) discusses several standard models of community organization including independent assortment, interspecific competition, and compensatory mortality. My results show that nymphal development overlaps between the melanoplines studied. In the foothills, M. sanguinipes and M. bivittatus nymphs development times overlapped. In the montane region, M. bivittatus and M. oregonensis development overlapped. Population sizes for these two species contrasted. At the 2250 m montane site, M. bivittatus numbers were low relative to M. oregonensis. while at the 2275 m montane site M. bivittatus numbers were larger while M. oregonensis numbers went down. It is not possible for me to make any conclusions about their food base, because I do not know what types of plants each of these species was utilizing. Mortality could be a factor influencing population sizes. M. bivittatus tends to be the larger of the two 51 species and may be more visible to predators. Lastly, one or the other of these species may be using a particular part of the vegetation structure that may affect its capture during sweeping. The only non-melanopline, C. curtipennis. appeared later in the growing season than the Melanoplines. It is a grass feeder and may be timing its life cycle to the grasses. Also the fact that C. curtipennis has four instars may allow it to be more flexible in areas with shortened growing seasons. These results present grasshopper community organization and phenology for two growing seasons. During this time, shifts in species presence/absence (ie. M- bivittatusl were observed at individual sites and may reflect the ephemeral nature to certain grasshopper populations. Grasshoppers in mountain environments are faced with conditions that in some cases reflect the limits of their geographic range, particularly in the alpine regions. Conditions such as frost after hatch, large variance in temperature, and short growing seasons are all thought to increase mortality at lower elevations. In the mountains, these conditions are typical throughout the summer months. It appears that the summer season in the mountains of this region are long enough to support one generation per year and that the compression of the life cycle enables grasshoppers to populate the montane and alpine zones. Long-term studies are required to assess the viability of these populations over time. 52 BIBLIOGRAPHY 53 Alexander, G. 1941. Keys Io1the identification of Colorado Orthoptera. Univer. Colorado Stud., Ser. D, 1:129-164. Alexander, G. 1951. The occurrence of Orthoptera at high altitudes with special reference to Colorado Acrididae. Ecology 32:104-112. Alexander, G. and J.R. Hilliard 1969. Altitudinal and seasonal distribution of Orthoptera in the Rocky Mountains of Northern Colorado. Ecological Monograph 39:385-431. Anderson, R.V. Tracey C.R. and Abramsky Z. 1979. Habitat selection in two species of short-homed grasshoppers (The role of thermal and hydiic stresses). Oecologia 38:359-374. Batschelet, E. 1981. Circular Statistics in Biology. New York: Academic Press. Blatchley, W. S. 1920. The Orthoptera of Northeastern North America. The Nature Publishing Company. 784 pp. Bodine, J.H. 1932. Hibernation and diapause in certain Orthoptera. HI. Diapause- a theory of its mechanism. Physiol. ZooL 5: 549. Brooks, A. R. 1958. Acridoidea of Southern Alberta, Saskatchewan, and Manitoba(Orthoptera). The Canadian Entomologist, Supplement 9, 92 pp. Capinera, J.L. and T.S. Sechrist 1982. Grasshoppers (Acrididae) of Colorado: identification, biology and management. Colorado State University Experiment Station, Fort Collins, CO. Bulletin No. 584S. Chappell, M.A. 1983. Metabolism and thermoregulation in desert and montane grasshoppers. Oecologia 56:126-131. 54 Chappell, M.A. and D.W. Whitman 1990. Grasshopper thermoregulation. In Chapman, R.F. and A. Joems (eds.). Biology of Grasshoppers. John Wiley and Sons, New York. ppl43-172. Daubenmire, R. 1988. Steppe Vegetation of Washington. Washington State University Cooperative Extension. Dingle, H. T.A. Mousseau and S.M. Scott 1979. Altitudinal variation in lifecycle syndromes of California populations of the grasshopper, Melanoplus sanguinipes (F.') Oecologia 84:199-206. Duman, J.G., K.L.Horwarth, A. Tomchaney, and J.L. Peterson. 1982. Antifreeze agents of terrestrial arthropods. Comp. Biochem. and Physiol. 73:545-555. Evans, E.W. 1988. Grasshopper (Insecta: Orthoptera: Acrididae) assemblages of tallgrass prairie: influence of fire frequency, topography, and vegetation. Can. J. Zool. 66: 14951501. Evans, E.W. 1984. Fire as a natural disturbance to grasshopper assemblages of tallgrass prairie. Oikos 43:9-16. Gage, S.H., M.K. Mukeqi, and R.L. Randell 1976. A predictive model for seasonal occurrence of three grasshopper species in Saskatchewan (Orthoptera: Acrididae). Can. Ent. 108:245-253. Gillis, J.E. and K.W. Possai 1983. Thermal niche partitioning in the grasshoppers Arphia conspersa and Trimerotropis suffusa from a montane habitat in central Colorado. Ecol. Entomol. 8:155-161. Gillis, J.E. and P.A. Smeigh 1987. Altitudinal variation in thermal behavior of the grasshopper Circotettix rabula (Rehn & Hebard) from Central Colorado. The Southwestern Naturalist 32(2):203-211. Grant, B. 1993. Alternative theory of grasshopper life cycles. Ecology 91:624-634. 55 Greg, S.A. 1963. The Ants of Colorado. University of Colorado Press, Boulder,Co. pp 34-66. Handford, R.H. 1961. Developmental patterns of the Clear-Winged Grasshopper at different altitudes and in different years on a sheep range in British Columbia, Canada. Can. Entomol. 93:665-670. Hebard, M. 1928. The Orthoptera of Montana. Proc. Acad. Nat. Sci. Philadelphia 80: 211-305. Heinrich, B. 1993. The Hot-Blooded Insects. Harvard University Press, pp. 143-167. Hewitt, G.B. and J.A. Onsanger 1983. Control of grasshoppers on rangeland in the United States: a perspective. Journal of Range Management 36:202-207. Janzen, D.H. 1973. Sweep samples of tropical foliage insects: effects of seasons, vegetation types, elevation, time of day, and insularity. Ecology 54:687-708. Joem, A. 1979. Resource utilization and community structure in assemblages of arid rangeland grasshoppers (Orthoptera: Acrididae). Trans. Am. Ent. Soc. 105:253-300. Joem, A. 1982. Vegetation structure and microhabitat selection in grasshoppers (Orthoptera: Acrididae). Southwest Nat. 27:197-209. Joem, A. 1982. Importance of behavior and coloration in the control of body temperature by Brachystola magna IGirardl (Orthoptera:Acrididae). Acrida 10:117-130. Kemp, W.P. 1986. Thermoregulation in three rangeland grasshopper species. Can. Entomol. 118:335-343. Kemp W.P. and N.E. Sanchez 1987. Differences in Post-diapause Thermal Requirements for Eggs of Two Rangeland Grasshoppers. Can. Entomol. 119:653-661 56 Kemp, W. P.; Kalaris, T. M.; and W. F. Quimby 1989. Rangeland grasshopper (Orthoptera: Acrididae) spatial variability: macroscale population assessment. I. Econ. Ent. 82:1270-1276. Kemp, W .P., Harvey, S.J. and K.M. O’Neill 1990a. Patterns of vegetation and grasshopper community composition. Oecologia 83:299-308. Kemp, W .P., S.J. Harvey, and K.M.O’Neill. 1990b. Habitat and insect biology revisited: The search for patterns. Amer. Entomol. 36:44-48. Kemp, W.P. and B. Dennis. 1991. Toward a general model of rangeland grasshopper (Orthoptera: Acrididae) phenology in the steppe region of Montana. Environ. Entomol. 20:1504-1515. Kemp, W.P. 1992. Temporal variation in rangeland grasshopper (Orthoptera: Acrididae) communities in the steppe region of Montana, USA. Can. Entomol. 124:437-450. Kemp, W.P. 1992. Rangeland grasshoppers (Orthoptera: Acrididae) community structure: A working hypothesis. Environ. Entomol. 21:461-470. Kingsolver, J.G. 1983. Thermoregulatory strategies in Colias butterflies. I. The determinants of body temperature and flight activity: elevational patterns and mechanistic limitations. Ecology 64: 534-546. Kingsolver, J.G. 1985b. Thermoregulatory significance of wing melanization in Pieris butterflies (Lepidoptera: Pieridae): physics, posture, and pattern. Oecologia (Berlin) 66: 546-553. Kingsolver, J.G. and W.B. Watt 1983. Thermoregulatory strategies in Colias butterflies: Thermal stress and the limits to adaptation in temporally varying environments. Am. Nat. 121:32-55. Lawton, J.H., MacGavin, M. and Heads, P.A. 1987. Effects of altitude on the abundance and species richness of insect herbivores on bracken. J. Anim. Ecol. 56: 147160. 57 May, M X . 1985. Thermoregulation. In: Comprehensive Insect Physiology,Biochemistry and Pharmacology. G.A. Kerkut and L.I. Gibert,eds., Pergamon Press, Oxford pp 507552. Marti, M.S. 1968. Ecology and Biogeography of High Altitude Insects. The Haugue. Dr.W. Junk N .V. Publishers. McCoy, E.D. 1990. The distribution of insects along elevational gradients. Oikos 58: 313-322. Moore, H.W. 1948. Variations in fall embryological development in three grasshopper species. Can. Entomol. 80: 83-88 Mueggler, W.F. and W.L. Stewart. 1980. Grassland and Shrubland Habitat Types of Western Montana. USDA Forest Service GTR INT-66. Mulkem, G. B. 1967. Food Selection by Grasshoppers. Ann. Rev. Ent. 12: 59-78. O’Neill, K.M. and W.P. Kemp 1990. Behavioral responses of the robber fly Stenopogon inquinatus (Diptera: Asilidae) to variation in the thermal environment. Environ. Entomol. 19:459-464. O’Neill, K.M., W.P. Kemp, and K.A. Johnson. 1990 Behavioral thermoregulation in three species of robber flies (Diptera: Asilidae: Efferiak Anim. Behav. 39:181-191. O’Neill, K.M. and W.P. Kemp 1992. Behavioral thermoregulation in two species of robber flies occupying different grassland microhabitats. J. Therm. Biol. 17:323-331. Otte, D. 1981. The North American Grasshoppers. Vol.l, Acrididae: Gomphocerinae and Acridinae. Harvard University Press. 275pp. Otte5D. 1984. The North American Grasshoppers. Voume II: Acrididae (Oedipodinae). Harvard University Press. 366pp. 58 Parker, M.A. 1930. Some effects of temperature and moisture upon Melanoplus mexicanus mexicanus Saussure and Camnula pellucida Scudder (Orthoptera). Montana Experiment Station Bulletin 223, 132 pp. Pfadt, R E . 1988. Field Guide to Common Western Grasshoppers. USDA APHIS, Wyoming Agricultural Experiment Station, University of Wyoming, pp I. Picket, S. T. A. and P. S. White (1985). The Ecology of Natural Disturbance and Patch Dynamics: An Introduction. Academic Press, New York. .472 pp. Price L. 1981. Mountains and Men: A Study of Process and Environment. Berkeley:University of California Press. 410pp. Rowell, C.H.F. 1971. The variable colouration of the acridoid grasshoppers. Advances in Insect Physiology 8:145-198. Salt, R.W. 1961. Principles of Insect Cold-Hardiness. Ann. Rev. Ent. 6:55-74. Samways, M.J. 1990. Land Forms and Winter Habitat Refugia in the Conservation of Montane Grasshoppers in Southern Africa. Conservation Biology 4:375-382. Scoggan, A.C. and M.A. Brusven. 1973. Grasshopper-plant community associations in Idaho in relation to the natural and altered environment. Melandria 12 : 22-33. Senft, R.L., Coughenour, M.B., Bailey, D.W ., Rittenhouse, L.R., Sala, O.E., and D.M. Swift. 1987. Large herbivore foraging and ecological strategies. BioScience 37: 789-799. Shreve, F. 1924. Soil Temperature as Influenced by Altitude and Slope Exposure. Ecology 5:128-136. Somme, L. 1989. Adaptations of terrestrial arthropods to the alpine enviroment. Biol. Rev. 64: 367-407. 59 ter Braak5 C.J.F. 1988. Canoco: an extension of Decorana to analyze speciesenvironment relationships. Vegetatio 75:159-160. Tolbert W.W., V.R. Tolbert and R.E. Ambrose 1977. Distribution5 Abundance,and Biomass of Colorado Alpine Tundra Arthropods. Arctic and Alpine Research 9:221-234. Uvarov5 B.P. 1931. Insects and climate. Trans Ent. Soc. London 79: 1-247. Uvarov5 B. P. 1977. Grasshoppers and Locusts, Vol. IL Center for overseas pest research: London Whitman5 D.W. 1987. Thermoregulation and daily activity patterns in a black desert grasshopper, Taeniopoda eques. Anim. Behav. 35:1814-1826. Willmer5P.G. andD.M. Unwin 1981. Field analysis of insect heat budgets: Reflectance, size, and heating rates. Oecologia(Berlin) 50:250-255. ###