n T
advertisement

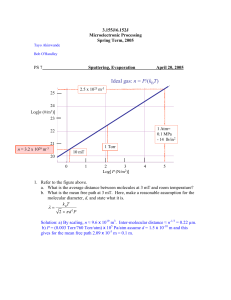
3.155J/6.152J Microelectronic Processing Spring Term, 2005 Tayo Akinwande Bob O'Handley PS 7 Sputtering, Evaporation April 20, 2005 Ideal gas: n = P/(kBT) 2.5 x 1025 m-3 25 24 Log[n (#/m 3)] 23 1 Atm= 0.1 MPa - 14 lb/in 2 22 21 n = 3.2 x 1020 1 Torr m-3 10 mT 20 0 1 2 3 Log[ P (N/m2)] 4 5 1. Refer to the figure above. a. What is the average distance between molecules at 3 mT and room temperature? b. What is the mean free path at 3 mT. Here, make a reasonable assumption for the molecular diameter, d, and state what it is. k BT λ= 2 × πd2P Solution: a) By scaling, n ≈ 9.6 x 1019 m3. Inter-molecular distance ≈ n-1/3 = 0.22 µm. b) P = (0.003 Torr/760 Torr/atm) x 105 Pa/atm assume d = 1.5 x 10-10 m and this gives for the mean free path 2.09 x 10-5 m = 0.1 m. 2. Enter “Sputtering”, Evaporation”, or “Both” below to best match the statement at left a. Viscous transport. __________________ b. Ballistic transport __________________ c. Film deposition rate depends on source vapor pressure. __________________ d. Film deposition rate depends on substrate bias & input power ________________ e. Film deposition rate depends on ratio of substrate area to source area. __________________ Solutions: Sputter, evap, evap, sputter, both. 3. When Ar+ ions strike the cathode, one of the results is the ejection of several electrons from the cathode. a. Explain what it is about the electrons that causes a “dark space” near the cathode? Answers: a) In order to cause the emission of a visible glow, the electrons have to accelerate to have a high-enough kinetic energy to cause an electronic transition greater than about 1.4 eV (red). They start out with nearly zero kinetic energy. 4. Mary’s lab partner, Bill, wants to grow an Al2O3 insulator film. Bill says he will us an Al target in a DC sputtering system and bleed in a continuous flow of O2 (at a rate he saw in the literature) into a pressure of 10 mT Ar. What should Mary tell him? Answer: Bill, you idiot, using a DC sputtering system will result in a buildup of charge on the Al anode. At some point the charge will reduce the voltage and quench the plasma. End of experiment. 5. It is very difficult to evaporate stoichiometric SiO2; you often get SiOx with 1 < x < 2, which implies a mixture of SiO and SiO2. But you still need to get as close as possible to SiO2. a) What partial pressure of O2 must you have in your vacuum chamber so that the flux of O2 on the substrate is the same as that of Si? Your evaporation source has a surface area of 1 cm2, the substrate is at a planetary radius of 20 cm, and your crucible is heated to 1500oC. b) What is the mean free path of O2 in this partial pressure (assume the diameter of an O2 molecule is 0.3 nm). c) What does your measurement in b) mean for your process? Solution . a) Acrucible = 1 cm2, r = 20 cm, TSi =1500°C (1773K) 2π kTSi mSi J O2 P = 1 = SiO2 × , mSi = 28 amu. mO 2 = 32 amu Acru J Si Peq.vap 2π kTH2 O m O2 4 πr 2 1500°C from table, PSi −3 ≅ 2 × 10 Torr 2 × 10 × [133(Pa Torr )]1cm −3 PO2 = b) λ = 4 π × 20 cm 2 2 2 293 × 32 1773× 28 −5 −7 = 2.3 ×10 Pa = 1.7 × 10 Torr kT = 23 cm (using T, P of Si) 2π d 2 PTot c) There are virtually no collisions between O2 molecules in the chamber, for that matter between O2 and Si vapor molecules. Both species do collide with walls of chamber. Without collisions, the two gases (Si, O2) are not in equilibrium. Also Si vapor follows a straight line path to substrate. It is only on the substrate that Si has a chance to interact with O2.