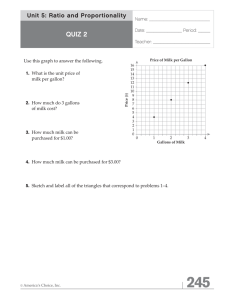
The autoxidative behavior of milk lipid fractions in systems of... by Shun Ku
advertisement

The autoxidative behavior of milk lipid fractions in systems of controlled composition by Shun Ku A thesis submittted to the Graduate Faculty in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE in Agricultural Products Utilization Montana State University © Copyright by Shun Ku (1966) Abstract: Fractions of milk lipids representing the phospholipid protein complex (fat globule membrane material from buttermilk (FGM I), and from butter serum (FGM II), the true fat (butteroil), and the intact milk fat globules (dialyzed cream) were allowed to autoxidize in systems composed of distillated water or milk serum or occasionally pH 6.60 phosptiate buffer. The effect of the following factors was investigated: (1) copper ion alone, ascorbic acid alone and copper ion plus ascorbic acid (2) sul-phydryl groups (L-cysteine), whole casein and K-casein (3) synthetic and naturally occurring antioxidants (NDGA and α-tocopherol). The autoxidative behavior of these milk lipid fractions was dependent on the particular fraction used and the nature and composition of the aqueous system into which it was dispersed. Copper ion alone and ascorbic acid alone, catalyzed oxidized flavor development by the fat globule membrane and dialyzed cream. Copper ion plus ascorbic acid gave the highest oxidized flavor intensity with fat globule membrane material. Copper ion from various salts gave variable catalytic power indicating that the catalytic power of copper ion in milk serum is dependent on the dominant kinds of cuprous salt complexes. The lowest oxidation intensities were given by the butteroil. The fat globule membrane from buttermilk (FGM I)? differed very significantly in its oxidative behavior in comparison to that from butter serum (FGM II). Generally FGM II gave much higher oxidation rates and intensities than FGM I. L-cysteine, whole casein, K-casein, NDGA and α-tocopherol behaved either as antioxidatants or pro-oxidants depending on the milk lipid fractions in question and the aqueous medium used. The reason for such behavior is discussed. THE AUTOXIDATIVE BEHAVIOR OF MILK LIPID FRACTIONS IN SYSTEMS OF CONTROLLED COMPOSITION by SHUN KU A thesis submittted to the Graduate Faculty in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE in Agricultural Products Utilization Approved: Head, Major Department MONTANA STATE UNIVERSITY Bozeman, Montana December, 1966 -iiiACKNOWLEDGEMENTS The author wishes to express his appreciation to Dr. A. M. El-Negoumy for his guidance and help through the investigation and the preparation of this thesis, and also his generous offer of prepared casein and Kappa casein. Thanks are also due to Dr. J. C, Boyd for the assistance enabling the author to pursue graduate work at Montana State University. Sincere appreciation is expressed to Dr. Graeme Baker for his useful suggestion in the author's experimental work. He also wishes to thank Dr. K. Goering, Dr. C. A. Watson and Dr. K. Emerson for helpful suggestions in setting his study program. The author wishes to express his sincere gratitude to his parents for their encouragement and understanding. I -ivTABLE OF CONTENTS Page VITA e o y o e e e e e e e e e e o e o o o e o 11 ACKNOWLEDGEMENTS . iii TABLE OF CONTENTS, iv v INDEX TO TABLES' ABSTRACT....... o * e « . e < e * e INTRODUCTION II. • ix e .......... ...................... 3 . . . . . . . . . . . 3 A. The Origin of Oxidized Flavor . . . . . . 3 B. The Role of Milk Fat and Phospholipids 5 .................. Nature of Autoxidation .......... Factors Influencing Development of Oxidized Flavor A. B. ............ . . . . . . . 8 Effect of Composition of the Aqueous Phase on Oxidized Flavor Development D„ 6 Effect of Copper, Sulfhydryl Group and Metal Protein Complexes . . . . . . . . . . . . . . C. 6 The Role of Copper in Presence and in Absence of Ascorbic Acid Effect of Antioxidants . . . . . . . 10 10 ............. I. Nordihydroguaiaretic Acid (NDGA) 2» Alpha-tocopherol............ . . . . . .... 10 11 . . . . . 12 Experimental Techniques . . . . . . . . . . . . . 13 A. 13 Ill. The Nature of the Fat Globule Membrane IV. I ... LITERATURE REVIEW. I. * The Use of Model S y s t e m ................ .. . -VB„ The 2-thiobarbituric Acid (TEA) Test .............. MATERIALS AND METHODS , , » , , e e * » » * e * * » o » * » * * * o @ * I. II. III. Preparation of Milk Lipid Fractions . . . . . . . . . . A. Fat Globule Membrane Materials (FQl) .............. B. B u t t e r o i l .................... ........... .. C. Dialyzed Cream (D.Cr.) . . . . . . . . . . . . . . . The Basic Medium For the Systems . . . . . . . . . . . . A. Milk Serum ........ . . . . . . . . . . . . . . . . B. Phosphate Buffer .................. C. Distilled Water . . . . . . . . . . . . . . . . . . . . . . . . . . Preparation of the Model Systems . . . . . . . . . . . . i IV. Measurements of Oxidation Rates and Intensity RESULTS AND DISCUSSION SUMMARY o e » e e APPENDIX LITERATURE CITED # e e ........................ e e e e e e e e o * o o e « , , , * @ * 0 * 0 » * * » » /"................ . - ‘ . . . . . . . . . . . . . . o o o e e o o o e * * » * » » » * * • -vi- INDEX TO TABLES Page Table I. II. III. IV. V. VI. VII. VIII. IX. X. The effect of Cu*-*" plus ascorbic acid on the autoxidation of various milk lipid fractions in different media (absorbance)........................... 25 The effect of increasing concentration of ascorbic acid in the absence of Cu++ on the autoxidation of milk lipid fractions (absorbance)......................... 62 The effect of increasing concentration of ascorbic acid in the presence of Cu++ on the autoxidation of milk lipid fractions (absorbance)......................... 63 The effect of Cu*""*" from various sources in the presence of ascorbic acid in water and milk serum systems on the autoxidation of milk lipid fractions (absorbance).................................... 64 The effect of varying fat content (from dialyzed cream) in presence of 4 ppm Cu++ and an increased concentration of ascorbic acid in milk serum on the autoxidation of milk lipid fractions (absorbance)..... 65 The effect of increasing concentration of L-cysteine in the presence of Cu++ and 2% of lipid on the autoxidation of milk lipid fractions (absorbance)......... 66 The effect of increasing concentration of casein in the presence of Cu++ on the autokidation of milk lipid,fractions (absorbance)......................... 67 The effect of increasing concentration of Kappa casein in the presence of Cu++ on the autoxidation of milk lipid fractions (absorbance)...................... 67 The effect of NDGA in the presence of Cu"*-*" on the autoxidation of milk lipid fractions (absorbance)......... 68 The effect of the antioxidant property of tocopherol on the autoxidation of milk lipid fractions (absorbance)..................j............ 69 -Vi,!Figure 1. Page The effect of increasing concentration of ascorbic acid in water system and in the absence of copper ions on the autoxidation of milk lipid fractions......... 25 2 . The effect of increasing concentration of ascorbic 3. 4. 5. acid in milk serum in absence of copper ions on the aytoxidation of milk lipid fractions..................... 27 The effect of increasing concentration of ascorbic acid in presence of 4 ppm Cu"*-*" in water system on the autoxidation of milk lipid fractions................. 28 The effect of increasing concentration of ascorbic acid in the presence of 4 ppm Cu^+ in milk serum system on the autoxidation of milk lipid fractions....... 29 The effect of Cu+* from different sources in water system on the autoxidation of milk lipid fractions....... 36 6. The effect of Cu*""*" from different sources in milk serum system on the autoxidation of milk lipid fractions......................................... 7» 8. .. The effect of varying fat content (from dialyzed cream) in the presence of 4 ppm Cu"*"*" and an increasing concentration of ascorbic acid in milk serum system on autoxidation of milk lipid fractions..... 38 The effect of increasing the concentration of L-cysteine in the presence of 4 ppm Cu"*""*" in water system on the autoxidation of milk lipid fractions................................................ .. 9. 10. 11. 37 The effect of increasing the concentration of L-cysteine in the presence of 4 ppm Cu*""*" in milk serum on the autoxidation of milk lipid fractions......................................... The effect of increasing concentration of casein in the presence of 4 ppm Cu+ *" in water system on the autoxidation milk lipid fractions............. The effect of increasing concentration of casein in the presence of 4 ppm Cu"*-*" in milk serum on the autoxidation of milk lipid fractions.............. ' 41 42 .. 46 47 -viiiFigures 12. 13. 14. 15. 16. 17. Page The effect of increasing concentration of K-casein in the presence of 4 ppm Cu++ in water system on the autoxidation of milk lipid fractions.................. 48 The effect of increasing concentration of K-casein in the presence of 4 ppm Cu++ in milk serum oh the autoxidation of milk lipid fractions.......... ........... 49 The effect of NDGA. in the presence of 4 ppm Cu++ in water system on the autoxidation of milk lipid f r a c t i o n s . . . 3 The effect of NDGA in the presence of 4 ppm Cu++ in milk serum system on,the autoxidation of milk Ixpid fractions........................................... 4 ^^ The effect of the antioxdant property of c(-tocopherol in wafer system on the auto­ xidation of milk lipid fractions........................ 55 The effect of the antioxidant property of of-tocopherol in milk serum system on the autoxidation of milk lipid fractions.......... . 57 -ix- ABSTRACT Fractions of milk lipids representing the phospholipid protein complex (fat globule membrane material from buttermilk (FGM I), and from butter serum (FGM II), the true fat (butteroil), and the intact milk fat globules (dialyzed cream) were allowed to autoxidize in systems composed of distillated water or milk serum or occasionally pH 6.60 phosptiate buffer. The effect of the following factors was investigated: (I) copper ion alone, ascorbic acid alone and copper ion plus ascorbic acid (2) sul= . phydryl groups (L-cySteine), whole casein and K-casein (3) synthetic and naturally occurring antioxidants (NDGA and o(-tocopherol). The autoxidative behavior of these milk lipid fractions was depen­ dent on the particular fraction used and the nature and composition of the aqueous system into which it was dispersed. Copper ion alone and ascorbic acid alone, catalyzed oxidized flavor development by the fat globule mem­ brane and dialyzed cream. Copper ion plus ascorbic acid gave the high­ est oxidized flavor intensity with fat globule membrane material. Copper ion from various salts gave variable catalytic power indicating that the catalytic power of copper ion in milk serum is dependent on the dominant kinds of cuprous salt complexes. The lowest oxidation intensities were given by the butteroil. The fat globule membrane from buttermilk (FGM I)? differed very significantly in its oxidative behavior in comparison to that from butter serum (FGM II). Generally FGM II gave much higher oxidation rates and intensities than FGM I . -X - L-cysteine, whole casein, K-casein, NDGA ando(-tocopherol behaved either as antioxidatants or pro-oxidants depending on the milk lipid fractions in question and the aqueous medium used. behavior is discussed. The reason for such INTRODUCTION Off-flavors resulting from lipid oxidation are among the most common flavor defects occurring in milk and its products. often described as: These off-flavors are cardboard, oxidized, metallic, oily, tallowy, or fishy depending on the intensity of the defect. This flavor defect causes millions of dollars of losses every year both to the milk producers and processors. The bulk, of the fat in milk is in the foi^n of small fat globules averaging 2-5 u in diameter. The surface of each fat globule is covered with a layer of material commonly known as the fat globule membrane (FQM). This, membrane is a complex made up mainly of phospholipids and proteins. From the standpoint of autoxidation, it is of importance to realize that these globules exhibit a large surface area which is rich in oxidizable phospholipids. The most important factors that tend to accelerate oxidized flavor development- are metal catalysis and the presence of ascorbic acid which provide a properly poised hydrogen accepting and donating system in the reduced-oxidized ascorbic, acid relationship. Research on oxidized flavor development in milk is complicated by the fact that different milks show.extreme variation in resistance and susceptibility. Some milks are objectionably oxidized within a few hours from milking, while in others, this effect cannot be induced even with large additions of copper. There is little doubt that the complexity and variability of milk composition are contributing factors to the confusing results sometimes obtained in oxidized flavor research. In the present investigation, model systems of known composition were used in an effort to minimize the variables. Various fractions of milk lipid were -2“ incorporated in these systems with the objective of determining the source of "off-flavors" and the compositional conditions that modify their in­ tensities. Among the lipid fractions used were: fat globule membrane . materials both from buttermilk, and butter serum; whole fat globules as dialyzed cream; butteroil prepared by skimming (devoid of phospholipids), and butteroil prepared by complete dehydration (rich in phospholipids)„ Comparative analyses were made to investigate the following: (1) The development and intensity of the flavor in various media such as milk serum, phosphate buffer and salt-free medium (water). (2) The effect of variation in the concentration of some of the major and minor milk constituents on the course and intensity of flavor development. (3) The role of copper ions and ascorbic acid in promoting or inhibiting oxidized flavor development in the various milk lipid fractions. (4) The effect of some antioxidants and the conditions leading to their relative effectiveness. REVIEW OF LITERATURE I. Ac THE NATURE OF AUTOXIDATION The Origin of Oxidized Flavor Oxidized flavor was first recognized as due to autoxidation of milk lipids by Rogers and Gray (1909). Greenbank (1940) believed this flavor was due to a group of unstable intermediate compounds which could be further oxidized to flavorless substances. The compounds resulting from lipid autoxidation represent a complex system, and the general routes of the reactions taking place were reported by Lea (1962) as follows: Dimer, Higher polymer t polymerization Autoxidation through free radical Fat Fat hydroperoxides further oxidation ✓ peroxides / ,/ polymers fission / hydration \ oxidation of CH=GH \ aldehydes ketoglycerides epoxides semi-aldehydes OH-glycerides aldehydo-glycerides Di-OH-glycerides OH-compounds acids El-Negoumy ej: a L (1962a) indicated that the polyunsaturated fatty acids, especially linolec and linolenic acid are the precursors for flavor compounds. According to Farmer and Sutton (1943b) and Farmer and Sundralingam (1943a), lipid autoxidation is initiated through a free radical mechanism as follows: -4- The initial step probably concerns the removal of hydrogen from a methylene group adjacent to a double bond. The resulting free radical may attach an oxygen to form a peroxide free radical which then abstracts hydrogen from another methylene group adjacent to a double bond to start over a formation of another free radical and continue the chain reaction. This mechanism was confirmed by chemical kinetic studies. (Holland and Gee 1946). The compounds responsible for oxidized flavor were reported to be carbonyls in nature and to include n-alkanals; 2-alkenals; 2, 4-dienals; ketones and vinyl ketones (Keeney and Doan 1951, a,b,c,; El-Negoumy et a l . 1961 and Tharp and Patton 1960). Malonaldehyde which is an intermediate compound in autoxidation and which is responsible for the 2-thiobarbituric acid color reaction, has been reported by Day (1960) to correlate well with organoleptic scores. Later research indicated that oct-l-en-3-one displays metallic flavor in oxidized dairy products with a flavor threshold of one part in q 10 in butter fat (Stark and Forss 1962) and that 2, 6 ,-nonadienal displays the grassy flavor (Hammond et: j*L 1964) . proposed as their respective Linoleate and linolenate were precursors. -5- Wilkinson (1964) suggested the following mechanism for the pro­ duction of oct-l-en-3-one through cleavage of the polyunsaturated fatty acid and secondary decomposition of the autoxidation products: CH3-(C H 2) 4-CH=CH-CH2- ^ CH=CH-CH2) 6 - C<>° 9 ,12-linoleic acid CH3(CH2) 4-CH=CH-CH2Oh oct-2-enol CHi(CH2)^-C H CH___ CH2 + +OH " intermediate CH3(CH2) 4-CH-CH=CH2 OH oct-l-en-3-ol through chain propagation or ^ hydroperoxides decomposition CH3(CH2) 4 -C - CH=CH2 +-H- & oct-l-en-3-one B, The Role of Milk Fat and Phospholipids From the standpoint of oxidized flavor development in aqueous media, only the 0.2-1.0% phospholipids in milk lipid (Jenness and Patton 1959) are of concern. The true fat (triglycerides) part of milk fat does not seem to be attacked in aqueous dairy products. The phospholipids which are present in the fat globule membrane are the site for oxidized flavor development (Palmer and Samuelsson 1924; Thurston ^t aL 1935). This is confirmed by the fact that the iodine number of the polyunsaturated ”6-* fatty acids in phospholipids was lowered substantially during oxidised flavor development while that of the triglycerides was practically unchang­ ed (Swanson and Sommer 1940)„ The vulnerability of phospholipids to attack by oxygen was proposed to be due. to their higher content of polyunsaturated fatty acids (Hilditch 1956; Smith and Lowry 1962) and also to their existence as a part of the phospholipid-protein complex of the fat globule membrane which is an ad­ sorption site for copper ions and ascorbic acid (Tarassuk and Koops i960)„ The carbonyl compounds ensuing from milk lipids during oxidized flavor development were isolated and characterized by several investigators Day and Lillard (I960), and El-Negoumy et al„ (1961), isolated them from autoxidized but ter oil, Forss et, aL» (1955, 1960 a,b,c,) isolated them from skim milk and washed cream, Bassetfe and Keeney (1960) isolated them from non-fat dry milk, while Parks and Patton (1961) isolated them from stored dry whole milk, autoxidized under various conditions„ Fhospholipids are preferentially oxidized in an aqueous system, while they serve as an antioxidant in non-aqueous dairy products (Patton 1962)o II. FACTORS INFLUENCING THE DEVELOPMENT OF OXIDIZED FLAVOR A. The Role, of Copper in Presence and in Absence of Ascorbic Acid The level of naturally occurring copper ions in milk is sufficient to catalyze the development of oxidized flavor (King and Dunkley 1959; Hunziker 1924). The addition of ascorbic acid tq metal-free milk retarded, but did not stop oxidized flavor development (Gorbelt and Tracy 1941). -I - On the other hand, in copper-contaminated milk, ascorbic acid is oxidized during storage and at the same time cupric ions are reduced to the cuprous state (Holmes 1953). Cuprous ions are the active catalyst in milk lipid autoxidation (Smith and Dunkley 1962 a,b). This mechanism was demonstrated by using the copper chelator, neocuproine and ascorbic acid oxidase. The following mechanism involving the catalytic role of the cuprous ions in peroxide radical formation was suggested by Wilkinson (1964): Hx /"X X/ CH-CH(OH) CH2OH + Cu++ X OH 2 Cu Z \ VX Q- / C = C O=C^ O=C CH-CH(OH)CH2OH + Cu+ \V 0 0 C / CH-CH(OH)CH0OH +HO2 - X 0 + H+ v WC ^\]H-CH(OH)CH-OH -8 Tarassuk and Koops (I960) suggested that a special structural con­ figuration of the copper-protein-lipid complex may be capable of attaching ascorbic acid to it permitting cupric ions to be converted to the cuprous state by ascorbic acid oxidation. The level of ascorbic acid has a direct influence on the autoxidation rate of milk fat globule membrane (King, 1962, 1963) , El-Negoumy (1965) suggested that both the oxidation rate and intensity in an aqueous phase are dependent pn the kind and predominance of the cuprous salt complexes formed„ B. ,I Effect of Copper. Sulfhydryl Groups and Metal Protein Complexes The sulfhy^ryl groups (-SH) in thioglycolic acid, cysteine and glutathione catalyze the autoxidation of phospholipids and unsaturated fatty acids (Meyerhof 1918, 1923; Harrison 1924; Hopkins 1925). This catalytic effect ceases when the (-SH) groups are oxidized to disulfide (-S-S-) or sulphate (Tait and King 1936). Catalysis of lipid oxidation involving (-SH) groups require the presence of traces of metallic ions, especially iron or copper, which also cafalyze the oxidation of (-SH) Hopkins (1925) suggested that (-SH) groups catalyze peroxide formation ■ , through their oxidation to the disulfide form (-S-S-)„ On the other hand, Forster and Sommer (1951) reasoned from their observation that trypsin re­ tarded or prevented oxidized flavor and that this was due to the release of reactive (-SH) groups from whey proteins„ This is confirmed by the fact that fishy flavor in washed cream was inhibited by addition of heated skim milk or whey and that the inhibiting effect was removed by blocking (-SH) with the SH inhibitor N-ethylmaleiamide (Tarassuk, Koops and Pette -9- 1959). Sulfhydryl compounds and proteins containing (-SH) groups are believed to be similar in their behavior to ascorbic acid during lipid autoxidation. Wilkinson (1964) proposed the following mechanism for explaining the role of cupric ions and glutathione during the course of peroxide formation by unsaturated fatty acids: R1 ,R" +2Cu++ S + SH+ Vs xi"" °, GSSG GSO2H x CU+ '' R' H ^ Xz H X / H / \ HgC C r " + 2 H- 2 RH \ / C / \ 2 H2C C-Rm (GS) I i! + 2 Cu \ H''' RO •, RO2, ROOH etc RH = Unsaturated fatty acid R = Glutamyl Radical R = Glycinyl Radical -10C. Effect of the Composition of the Aqueous Phase on Oxidized Flavor Development The composition of the aqueous medium in which the lipid is dispers­ ed has a very significant effect on both the rate and intensity of oxidized flavor development by milk fat. The various salts present in milk serum may form complexes with ionic copper, which makes them vary significantly in their catalytic power (El-Negoumy 1965). El-Negoumy (1965) also found the concentration of sodium caseinate, ©(-lactose and ascorbic acid to significantly influence autoxidation of globular milk fat. This effect was dependent on the kind and concentration of other solubles in the system. D. Effect of Antioxidants I . Nordihydroguaiaretic Acid (NDGA) Nordihydroguaiaretic acid (NDGA), a phenolic antioxidant has the following structure: Its antioxidant properties result from the two hydroxyl groups in the ortho and para configuration (Matill 1931; Stull ej: a L 1948 a, b) . A kinetic study (Lundberg at al.1947a) indicated that in general, the loss of antioxidant property of phenolic antioxidant in autoxidized fats did not occur as a single reaction but was completely dependent on reaction with the products of fat oxidation. NDGA was found to be an effective antioxidant for various dairy products such as frozen sweet cream (Stull at aL 1947, 1949), whole -11milk and unsweetened frozen creep (Stull et al. 1948a), spray-dried milk and ice crgam (Stull £t al. 1951 a, b) , butter (Krukovsky j2t al. 1949 a,b) , butferoil (Wyatt an4 Day 1965), and salted sweet cream butter (El-Negoumy and Hammond 1962). NDGA functions as an antioxidant by retarding oxygen absorption by phospholipids and methionine. Ascorbic and citric acids act as syner­ gists which enhance the effectiveness of NDGA. This antioxidant shows its greatest action near pH 6.5 (£>tull ej: al. 1951b) . NDGA retards the induction period of metal catalyzed lipid oxidation (McDowell, 1955). Non-oxidized and stale flavors were reported in cold storage dairy products containing NDGA (El-Negoumy and Hammond 1962; Wyatt and Day 1965). Thfe optimum concentration of NDGA for its most effective antioxygenic aption lies within 0.001 to 0.01% (Stull jit al.1951b). 2. Alpha-tocopherol Tocopherols (vitamin E) arfe well known as antioxidants in animal tissues (Zalkin et al.1960; Edwin et al.1961). Alpha-tocopherol appears to be a naturally occurring antioxidant in milk fat which contains about 0.022 mg/g. (Webb and Johnson 1965), A significant correlation exists between the tocopherol content of milk and its ability to resist oxidized flavor development (Krukovsky et al.1949'a.b). Natural alpha-tocopherol occurs in the unsaponificable material of milk fat and can be added to the extracted fat to enhancfe its stability (Smith et al,1948). • 'I Addition of alpha-tocopherol to milk during cold storage at con­ centration from 0 .2-2 .4 mg per liter decreases the loss of reduced ascorbic acid and increases somewhat the milk's resistance to oxidized flavor "12development (Holmes 1952). III. THE NATURE OF THE FAT GLOBULE MEMBRANE The bulk of the lipid material in milk exists in the form of an emulsion of tiny spherical particles (3-5 u in diameter), or globules dispersed in milk serum (Jenness and Patton, 1959). A membrane surround­ ing each fat globule was demonstrated microscopically by Storch in 1897, The phospholipids occur in the membrane as a monomolecular layer which is associated with the membrane protein iri the form of a complex (Palmer and Samuelsson, 1924; Palmer and Wiese, 1933; Sommer, 1951; and Brunner et al. 1953 a,b). King (1954) visualized the fat globule membrane to be made of a surface layer of polar phospholipids, cholesterol, and vitamin A which is associated with the high melting triglycerides in the lipid phase of the fat droplets and are attached to the protein in the aqueous phase of milk serum. Morton (1954) reported the fat globule membraine to be an accumu­ lation of lipoptotein particles, "milk microsomes", which passed into the m|.lk from the mammary gland during milk secretion. Brtinner (1962) considered Van der Walls force, hydrogen bonding, and electrostatic attraction to be the major forces responsible for proteinlipid interaction in the membrane. Harwalker and Brunner (1965) suggested that the hydrophobic and covalent disulfide bonds may be significant forces in the microstructure of the membrane complex. . The milk fat globule membrane was suggested as a possible site of lipid oxidation due to; (a) its high oxidizable phospholipids content (Jenness and Patton, 1959); (b) its high catalytic copper content (Herald -13- et aL 1957b and Ramachandran et al. 1960) Herald and Brunner (1957a) isolated and separated ,the fat globule membrane proteins into two fractions based on their solubility in 0 .02M NaCl„ The insoluble fraction had a brown reddish color; properties simi­ lar to those of pseudokeratin (Brunner 1962) and a higher density than the soluble fraction. These workers also found the insoluble fraction to be more tightly associated with the fat globules (Jackson and Brunner 1960) I than the soluble fraction. On the other hand, the soluble fraction had a low-density, whitish color, properties similar to glycoprotein (Brunner 1962), and higher phosphorus content than the insoluble fraction (Herald et a I. 1957b) . IV. A. EXPERIMENTAL TECHNIQUES The Use of Model System Model systems are used for the purpose of investigating the effect ' of various milk constituents on the development of oxidized flavor. King (1962) used model systems composed of pH 6.5 phosphate buffer to investi­ gate the autoxidation of fat membrane material in presence of ascorbic acid. El-Negoumy (1065) investigated dialyzed globular milk fat in the presence of various salts and in both water and phosphate buffer systems. The 2-thiobarbituric acid test was used in King's (1962) and El-Negoumy1s (1965) work to measure the oxidation rates. Reaction con­ ditions were standardized by King (1962) using model systems. B. The 2-thiobarbituric Acid (TBA) Test 2-thiobarbituric acid (TBA) has the following structure: -14- TBA reagent was first used by Liversedge and Kohn (1944). They observed that various animal tissue slices, after incubation under aerobic conditions, developed a characteristic color which was measured spectrophotometrically at 535 mu. The color was produced as a result of the interaction of products of oxidation of unsaturated fatty acids with TEA. Dunkley and Jennings (1951) found that the absorbance of the TBA color reaction correlated closely with the organoleptic flavor score of oxidized milk. Several reports (Patton £t aJ.. 1951 and Biggs and Bryout 1953) discussed the usefulness of this reaction and factors influencing the results, such as the pH, heating time, method of measuring color, and copper contamination. Sidwell et al.(1954) compared the peroxide value, total carbonyl value and TBA test in the same samples and found them to be correlated with each other. Jennings £t al. (1955) found the spectra of pigments derived from the TBA reaction with oxidized milk to be identical with those derived from TBA-malonaldehyde reaction. Sinnhuber And Yu (1958) used elemental analysis to determine the empirical formula of TBA pigment as C ^ H g N ^ O ^ ^ . Furthermore, the of one pigment derived from TBA-oxidized milk and the pigment derived from TBA-malonaldehyde on paper chromatography were identical. They believed -15- the pigment to be a condensation product produced according to the following reaction: HS S^/N^jOH 1K z O H x: -CHo-C^ / \ H + 2 H2O HCl H2O tl ^iT I= CH-CH=CH (pigment) O H The exact mechanism of maIonaldehyde formation during autoxidation is not known. Wilkinson (1964) suggested that the precursors of malondaldehyde may be derived from linoleates-2-enals and 2, 4, dienal as follows: R - CH 2 -CH=CH-CHO °2 + Cu++ Z R - GH -CH=CH-CH=O t acid + Cu++ 0 i R - CH=O + CH(OH)=CH-CH=O malondaldehyde (enol form) According to King (1962), the use of trichloroacetic acid in the TBA test has several advantages. Trichoroacetic acid is used to flocculate the proteins and provide the acidity necessary to carry out the subsequent reaction with TBA. This high acidity effectively stops lipid reactions which are particularly important when applying such a rapid raction to milk. They also found that lactose interferred with the TBA reaction. This was shown by chromatographic separation and spectrophotometrie analyses of the TBA pigments. A satisfactory color formation was -16— indicated at 60°C at which lactose degradation is minimized. Results were also presented, showing good correlation of the oxidized flavor with the TBA test. King (1962) also found the TBA test to correlate well with organoleptic judgment. MATERIALS AND METHODS I. Preparation of Milk Lipid Fractions A 0 Fat Globule Membrand Materials (FQl) The fat globule membrane material was prepared according to King (1962)„ Fresh raw cream obtained from the University's dairy plant was warmed to 40°C and washed four times, using four folds of its voluthe of tap water with a De Laval Model 518 cream separator. to remove all traces of skim milk constituents. The objective was The washed cream was then cooled to S-IO0C s and churned in a laboratory churn to obtain butter arid buttermilk. The pH of the buttermilk was adjusted to pH 4.9 with 0.1 N HCl followed by exhaustive dialysis against pH 4.9 water for 48 hours at 2-4 C . The dialysis water was changed every 12 hours. The volume of the dialyzed buttermilk was reduced considerably by perevaporation, and the balance of the water was then removed by freeze drying. has been designated ,FGM I . i This preparation The butter was melted at 70°C and then sep- arated into butteroil and butter serum using a separatory funnel. The butter serum was treated in the same manner as the buttermilk to obtain a preparation designated as FQl II. of these preparations; The following is a schematic diagram -18Raw Cream Warm to 40°C add tap water and separate Repeat 4 times \l/ Washed Cream Cool overnight at 5-10°C v Churning / Buttermilk V Butter Melt at 70°C Butteroil Butter serum 4 pH adjusted to 4.9 (Treat as Buttermilk) s/ Dialyzed gainst pH 4.9 water for 48 hours Perevaporate 4 Lyophilize 4 FGM I (from buttermilk) B. FGM I I (from butter serum) Butteroil The methods used in preparing the various butteroils were those used by El-Rafey £t jal. (1944). I. Phospholipid rich butteroil (B.O.I): Melted butter was saturated with NaCl and the moisture was removed by boiling. The proteins and membrane material were precipitated by NaCl, leaving a golden yellow butteroil which was separated by a separatory funnel to remove all contaminants. did not exceed IlO0C. The processing temperature -19- 2. Skimmed, 70°C, butteroil (B.O.II): Butter was melted at 70°C, which resulted in separation of the butteroil layer from the butter serum. The butteroil was skimmed off then filtered through a layer of glass wool. This butteroil is almost devoid of phospholipids. 3. Butteroil III, rich in phospholipids heated to 130°C (B.O.III): The butter was melted and heated with continuous stirring at IOS-IlO0G until the water had been evaporated and the non-fat solids settled. The temperature was then raised to 130°C. As the temperature approached 120°C, the butteroil formed a rela­ tively stable foam which dissolved back into the fat on cooling. The fat had a dark reddish color and the characteristic aroma of the butteroil prepared by the boiling-off process. addition, it had a better flavour and odor. In The fat was then clarified by centrifuging and the dark brown sediment was removed. C . Dialyzed Cream (D.Cr.) Fresh raw cream was washed with tap water and then dialyzed for 48 hours. water,. The fat content was then adjusted to 30% by adding distilled It was then divided into 150 ml portions which were placed in plastic bags and frozen until needed. II. A. THE BASIC MEDIUM FOR THE MODEL SYSTEMS Milk Serum Milk serum was prepared by dialyzing 2 liters of 5% lactose solution against 24 gallons of skim,milk for 48 hours at Z-S0C . The milk serum was - 20 - then divided into small portions, frozen and used as needed„ B. Phosphate Buffer ■ ! Phosphate buffer (pH 6 .6) was prepared by dissolving 4.4 g NaH2P04 ' plus 2.6 g NagHPO^ per liter of distilled water. C. 1 Distilled Water Fresh prepared distilled water was used. Ill. PREPARATION OF THE MODEL SYSTEMS All the model systems used in this investigation contained 2% lipid material and 4 ppm copper ions per 100 ml. These systems were basically made of either distilled water or milk serum. Distilled water and phos­ phate buffer were used for comparative experiments. The other components in these systems were added at the concentrations indicated in the individI ual experiments. All systems were adjusted to pH 6 .6-6.8 using either 0.10 N HCl or NaOH as needed. Oxidation was allowed to proceed for 48 hours at 3-5°C before analysis. Whole casein and K-casein were prepared by Dr. El-Negoumy. Whole casein was iso-electricalIy precipitated from fresh skim milk at pH 4.60 and separated by centrifugation at 3000 x g. This wet casein was washed 5 times with distilled, water, with the aid of an electric blender. The washed casein was then freeze dried. K-casein, prepared from whole casein was purified by the method , of Zittle and Custer (1963). ' 'I I Ascorbic acid," L-cysteine, NDGA, and o(-tocopherol were commercial preparations purchased frop Nutritional Biochemicals Corporation, Cleveland, Ohio. -21IV. MEASUREMENT OF OXIDATION RATES AND INTENSITY Progress of oxidized flavor development was followed by the 2-thiobarbituric acid test, using King's method (1962) as follows: The oxidized material (17.6 ml) were pipetted into a glass stopper­ ed flask. After warming to 30°C, 1.0 ml of 100% trichloroacetic apid solution was added, followed by the addition of 2.0 ml of 95% ethanol. The flask was stoppered and shaken vigorously for 10 seconds. After J 5 minutes, the contents were filtered through Whatman No. 42 filter paper. ; To 4.0 ml of the clear filtrate 1.0 ml of TBA solution was added. The TBA solution was made by dissolving 1.4 g 2-thiobarbituric acid in 100 ml of 95% ethanol. The flask Was stoppered, mixed and placed in a 60oC water bath for one hour. I The reaction solution was cooled to room temperature ;■ . - - and its absorbance measured at 532 mu using a Beckman Model B spectrophoto­ meter. Blank determinations were made with each set of experiments using fresh identical systems which were not oxidized. The absorbance of the blanks were subtracted from those pf the samples tp give the absorbance due to lipid oxidation. RESULTS AND DISCUSSION In the present investigation, various fractions of milk lipid were subjected to autoxidation in model systems composed basically of distilled water, or milk serum, and occasionally pH 6.60 phosphate buffer. Among the milk lipid fractions, the phospholipid-protein complex of milk fat was represented by the fat globule membrane material and whole globular milk fat was represented by dialyzed cream. Pure butteroil represents the true fat (triglycerides) of the milk fat globule. Six milk fat preparations were used in this investigation: (1) Fat globule membrane prepared from buttermilk (FGM I): This was a brown reddish color, a high density and retained a hygro­ scopic consistency upon freeze drying. (2) Fat globule membrane prepared from butter serum (FGM II): This has a whitish appearance and a lower density than FGM I . (3) Dialyzed cream (D.Cr.): This was prepared from washed cream by exhaustive dialysis. (4) Butteroil I (B.O.I): Rich in phospholipids, prepared by the boiling-off method at IlO0C . (5) Butteroil II (B.O.II): Devoid of phospholipids, prepared by the floatation method at 70°C. (6) Butteroil III (B.O.III): Rich in phospholipids, prepared by the boiling-off method at 130°C. The difference in physical appearance and properties of FGM I and FGM II is probably due to differences in the amounts, and possibly the kinds of proteins, which make up the phospholipid-protein complex. Herald ej: al. (1957) separated 2 protein fractions from the fat -23- globule membrane based on their solubility in 0.02 M NaCl. One of them was insoluble and generally had an appearance similar to FGM I prepared here, while the soluble protein was similar to FGM II. These authors indicated that the soluble fraction had a higher copper and phosphorus contend: than the insoluble fraction. An examination of the results obtained here with these 2 fractions indicated that they generally differed substantially in their oxidative behavior. Generally FGM II exhibited much higher oxidation intensities than FGM I. The butteroil preparations showed the lowest oxidation intensities and rates, probably because they lacked the fat globule membrane. Dialyzed cream, which is composed of the triglycerides and surrounded by the intact fat globule membrane, showed oxidation rates and intensities between those given by the globule membrane and butteroil. The rates and intensities of oxidized flavor development were measur ed in all model systems in terms of the absorbance of the 2-thiobarbituric acid (TEA) color reaction. The negative absorbance value indicate that it was lower than that of the blank test. (I) The fat globule membrane: An examination of the data presented in Table I and Figures I, 2, 3, and 4 reveal that the fat globule membrane materials (FGM I and FGM II) gave the highest oxidation rates and intensity in the presence of ionized copper alone, ascorbic acid alone or ionized copper plus ascorbic acid in comparison to other milk lipid fractions. This is undoubtedly due to their higher contents of phospholipids and polyunsaturated fatty acids which make them the probable site for oxidized -24flavor development in aqueous systems„ This is supported by the data obtained by Swanson and Sommer (1940) who found the iodine number of the fatty acids located in milk phospholipids to be lowered substantially through autoxidatioh, while that of the triglycerides remained practically unchanged. Milk phospholipids have been reported ^ich in polyunsaturated fatty acids by Hilditch (1956) and Smith and Lowery (1962). The polyun­ saturated fatty acids, especially linoleic and linolenic acids were shown by El-Negoumy et: al^. (1962a) to be the precursors for oxidized flavor compounds in milk fat. The FGM material is also richer in its natural copper content than whole milk (Herald et al. 1957b). In the presence of copper alone, the oxidation intensity given by FQl II in pH 6.60 phosphate buffer was almost double that given by FQl I (Table I). This behavior is indicative of the compositional differences between the 2 preparations. The difference may be the result of a higher concentration of polyunsaturated fatty acids in FQl II. Verification of this point awaits future investigations. Ascorbic acid alone, in the absence of copper, exhibited powerful pro-oxidant properties in the presence of the fat globule membrane material (Table I and Figures I, 2). This behavior is, in all probabilities, due to the higher natural copper content of the metallo-phosphilipid-protein comp­ lex of the fat globule membrane. Herald et al. (1957k) reported that the fat globule membrane material contained a higher concentration of mineral elements than milk serum. They suggested that these elements are possibly constituents of the various enzymes in the membrane. Ramachandran et: al. (I960) showed the fat globule membrane to be rich in copper and iron. TABLE NO. I The Effect of Cu1-*" Plus Ascorbic Acid On The Autoxidation Of Various Milk Lipid Fractions In Different Media (absorbance) Milk Lipid Fractions Reaction Medium Distilled Water Cu+1- Ascorb­ ic Acid Cu^+ As.A. Phosphate Buffer (pH 6.60) Cu h " Ascorb­ Cu"""+ ic Acid A.A. Milk Serum Cu h " Ascorb­ ic Acid Cu h + As.A. I tsd Vi FQM I (from buttermilk) 0.228 0.125 0.565 0.270 0.290 0.720 0.-242 0.157 0.317 FGM II (from butter serum)' 0.217 0.477 0.477 0.430 0.135 0.835 0.298 0.181 0.478 Dialyzed Cream 0.042 0,045 0.177 0.092 0.128 0.195 0.105 0.155 0.310 B.O. I 0.014 0.024 0.053 0.001 0.014 0.031 0ioi7 0.017 0.026 B.O. II 0.006 0.008 0.040 0.006 0.018 0.106 0.020 0.013 0.042 B.O. Ill 0.015 0.008 0.017 0.004 0.004 0.035' 0.010 0.011 0.032 i -26- ,------ FGM I #-— ■ — — < FGM TI TBA (Absorbance) ^iimiwiiu i D i a l y z e d Cream Ascorbic Acid (mg/100ml) Figure No. I: The effect of increasing concentration of ascorbic acid in water System in the absence of copper ions on the autoxidation of milk lipid fractions. -27- FQi I FGM II .« Dialyzed Cream B.O. I B .0. II (Absorbance) B.O. Ill Ascorbic Acid (mg/lOOml) Figure No. 2: The effect of increasing concentration of ascorbic acid in milk serum in absence of copper ions on the autoxidation of milk lipid fractions. -28- .-------- . FGM I ♦— — — — & FGM XX Dialyzed Cream B.O. I B .0. II (Absorbance) B.O. Ill Ascorbic Acid (mg/lOOml) Figure No. 3: The effect of increasing concentration of ascorbic acid in presence of 4 ppm Cu++ in water system on the autoxidation of milk lipid fractions. -29- 4 FGM minimi .<./)/ „ Dialyzed Cream B .0. I B.O. II TBA (Absorbance) B.O. Ill 8 12 16 Ascorbic Acid (mg/100ml) Figure No. 4: The effect of increasing concentration of ascorbic acid in the presence of 4 ppm Cu++ in milk serum system on the autoxldation of milk lipid fractions. -30= Richardson and Guss (1965) found the high density membrane material to contain one atom of copper or iron per 21 to 24 molecules of polyunsaturated fatty acidso The development of oxidized flavor by the fat globule membrane in the absence of copper ions and in the presence of ascorbic acid alone offer an explanation for development of this flavor in milk, free from metallic contamination. It is a well known fact that the elimination of copper ions completely from dairy processing equipment did not give milk supplies much protection against this defect. As a matter of fact, bulk milk supplies seem to be more susceptible to development of oxidized flavor in recent years due to natural copper. FQl I and FGM II differed very significantly in their oxidative behavior in the presence of ascorbic acid alone. The high oxidation intensity given by FQl II in a salt free system (water) is again a proof that these 2 membrane preparations a.re quite different from each other. This is probably the result of the higher content of polyunsaturated fatty acids and natural copper in FQl II. It is suprisipg, however, that FQl I reversed its oxidative trend in water and gave a higher oxidative intensity than FQl II in pH 6.6 phosphate buffer. ' : This behavior may bd due to differences in the configuration of the . . . copper-protein-lipids complex in FQl I and FQl II. It is possible that FQl I is more able to promote the formation of the catalytic cuprous phos­ phate complex. The copper-protein-lipid complex of the fat globule membrane have been reported by Tarassuk and Koops (I960) to attach ascorbic acid. -31- In the presence of ionic copper plus ascorbic acid, the oxidation intensities given by both FGM I and FfiM II are close in all systems„ The highest oxidation intensity of FGM preparations was in phos­ phate buffer, followed by water, and then milk serum. Cuprous phosphate complex has been shown by El-Negoumy (1965) to be a more powerful catalyst for oxidized flavor development than other cuprous complexes. An increas­ ed concentration of ascorbic acid in the absence of copper ions (Figures I and 2) in water systems induced an oxidation intensity with FQl II which was double that given with FQl I . Both of the membrane preparations, however, increased their oxidation intensity significantly with an increase in ascorbic acid concentration. Thg same trend was followed in milk serum systems, except that the intensity was generally lower than that in water systems. The salts present in milk serum probably interact with copper, occurring naturally in the membrane material, forming cupric and cuprous I complexes. ; The catalytic effect of copper will be dependent on the kind and amount of anions in these complexes. According to Smith and Dunkley (1962a), cuprous ions are the active form initiating the oxidation reaction. Thus, the predominance of the cupric salt complexes in milk serum could be responsible for the lowered oxidation intensity observed in this medium. Another possible explanation for this behavior in milk serum could be the configurational changes occurring in the natural copper-protein-phospholipid complex of the membrane Aaterial as a result of interaction with the salts in the milk serum. Tarassuk and Koops (I960) suggested that naturally occurring copper exists as a copper-protein complex which is capable of accepting or attaching ascorbic acid upon reduction of cupric to cuprous j -32- ions O The highest oxidation rates and intensities were given by both preparations of the membrane material in the presence of copper plus ascorbic acid (Table I and Figures 3 and 4). phosphate buffer and lowest in milk serum. The intensity was highest in The work of Freiden (1958a), Freiden and Alles (1958b), Kelley and Watts (1957), Filtman and Freiden (1957) and King and Dunkley (1959) showed that ascorbic acid causes rapid reduction of cupric to cuprous copper. The abundance of the cuprous ions is especially manifested in phosphate buffer where the cuprous complex formed is highly pro-oxidant. This is in agreement with the findings of El-Negoumy (1965) who reported the cuprous salt complex formed with phos­ phate to be twice as effective in its catalytic effect as those formed in the presence of sodium citrate. An increased concentration of ascorbic acid in the presence of copper ions gave an oxidation intensity which was much higher in water > . than in milk serum systems (Figures 3 and 4). This is in agreement with the findings of King (1963). Here again the copper salt complexes formed in the presence of milk serum had an overall suppressive effect on adtoxidation in comparison to salt free systems (water). The presence of copper chelators such as sodium citrate in milk serum and the predominance of cupric salt complexes is most likely responsible for this behavior. (2) The intact globular milk fat in dialyzed cream; The oxidation intensity developed by dialyzed cream in the presence of copper alone was dependent on the composition of the aqueous system in which it was dis­ persed (Table I ) . Water systems gave the lowest intensity, followed by -33- Phosphate buffer, and then milk serum. This is in agreement with the results of El-Negoumy (1965) who suggested that cuprous ions initiate the autoxidation reactions, only when they exist in the form of complexes with anions in the system. The higher oxidative trend of dialyzed cream in phosphate buffer and milk serum points to the validity of this sugges­ tion. Ascorbic acid alone initiated oxidation of globular fat, especially in phosphate buffer and milk serum. This behavior is probably due to the presence of the intact membrane material surrounding each fat globule. The meribrane material, because of its high content of phospholipids and natural copper is probably the site of oxidized flavor development in the intact fat globules. Nelson and Pink (1954) and French and Monks (1961) presented evidence indicating that copper carboxylate complexes in organic compounds exhibit considerable ionic character. This, if true, means that natural copper in the metal-lipo-protein complex is capable of ionization which would increase its catalytic power considerably. Ascorbic acid is capable of reducing natural copper to the cuprous state according to Smith and Dunkley (1962 a,b). The results obtained with increasing concentration of ascorbic acid in water systems where a prooxidant effect is evident supports this conclusion. Smith and Dunkley (1962) have also shown a strong positive correlation between the incidence of a spontaneous oxidized flavor development and the high levels of natural copper in milk. The low oxidation intensity of an increased concentration of ascorbic acid alone in milk serum is probably due to the reduction in the catalytic power of copper salt complexes in comparison to the cuprous *34— ascorbate complex. Free ascorbic acid exhibits antioxidant properties. The intact globular milk fat gave its highest oxidation intensity in the presence of copper plus ascorbic acid (Table I and Figures 3 and 4). Disregarding differences in the levels of oxidation intensities, this trend is similar to that given by the fat globule membrane in presence of copper ions plus ascorbic acid. This similarity is probably a reflection of the presence of the fat globule membrane. An increased concentration of ascorbic acid in the presence of copper ions gave an increased oxidation intensity in water systems, with I the reverse trend being true in milk serum systems (Figures 3 and 4). Results similar to these were obtained by El-Negoumy (1965) in salt free systems and in systems containing individual salts or their mixtures. He suggested that, in the absence of salts, reduced ascorbic acid converts cupric to cuprous ions in amounts proportional to its concentration. cuprous ascorbate acts as a strong peroxidation catalyst. The In milk serum, the cuprous ions complex with the salt anions, thus sparing the ascorbic acid which then behaves as an antioxidant, especially at higher concentra­ tions » Copper ions (4 ppm) from various salts gave variable rates and in­ tensities in similar systems containing dialyzed cream and an increasing concentration of ascorbic acid (Figures 5 and 6). All copper salts gave a pronounced pro-oxidant effect in water systems with an increased concenx , tration of ascorbic acid. On the other hand, no significant changes in oxidation rates or intensity occurred in milk serum systems. These results are in support of El-Negoumy1s suggestion (1965) that the catalytic power -35- of copper induced oxidized flavor development in milk is dependent on the kind and relative predominance of the various anions which complex with cuprous ions. Copper in the presence of acetate, carbonate and nitrate anions had less catalytic power than the sulfate in water systems„ Smith and Bunkley (1962 a,b) suggested that cuprous copper in the form of corns? plexes is the limiting peroxidation factor which promotes spontaneous oxi­ dized flavor development„ According to Wilkinson (1964), the ionic species depicted by many investigators to be free in autoxidation, is more likely to exist as a complex or chelate in an aqueous medium. The results ob­ tained here, with the various copper salts, support Wilkinson's suggestion. Both the rate and intensity of oxidized flavor development by ■ -•. intact globular fat are significantly influenced by the fat content of the system. This is clearly demonstrated by the data in Figure 7. The reason for the observation that concentrations higher than 0.50 mg ascorbic acid are antioxidants in systems containing 2-4% fat, and pro-oxidant in systems containing 6-12% fat is probably du.e to the abundance of fat globule membrane material in the latter. The antioxidant protection given at higher concentrations of ascorbic acid is much less apparent in systems containing higher fat content because of the presence of more substrate for oxidation. The variation in oxidation intensity observed at higher fat concentrations is probably due to the accumulation of larger amounts of oxidation products which usually suppress autoxidation after ,reaching ‘ | ’• i ' a certain concentration. , ' I’ The oxidation products presumably do this by intercepting and interacting with the free radicals necessary for initiat­ ing the reaction. King (1963) suggested that the milk lipfd oxidative -36- Cu (CH3COO)2 CuCO CuSO, TBA (Absorbance) 0.4 - 8 12 16 Ascorbic Acid (mg/100ml) Figure No. 5: The effect of Cu++ from different sources in water system on the autoxidation of milk lipid fractions. -37- (Absorbance) Cu(CH3COO)2 Figure No. 6: The effect of Cu4+ from different sources in milk serum system on the autoxidation of milk fractions, -38— x__. TL fat , 47, fat 67, fat 1.0 ►— Z — — —— * 8% fat »--------- »10% fat Hist.....,.*.**"*#12% fat 0.8 « •l (Absorbance) V-1*" 0.6 ....'''X S °*4 •V< 0.2 8 12 16 Ascorbic Acid (mg/lOOml) Figure No. 7: The effect of varying fat content (from dialyzed cream) in the presence of 4 ppm Cu++ and an increasing concen­ tration of ascorbic acid in milk serum system on autoxudation of milk lipid fractions. -39- system is activate^ by association with ascorbic aci.d and inhibited by the products of lipid and ascorbic acid oxidation. El-Negoumy (1965) suggested that at higher ascorbic acid concentrations, the lipids are spared by inter­ actions between the degraduation products of both the ascorbic acid and lipid oxidation and cuprous, ions. This reduces the cuprous ions available for initiating the reaction. (3) The true fat or triglycerides (Butteroil): Virtually no oxi­ dation took pl,ace in the 3 butteroil preparations in the presence of copper alone or ascorbic acid alone (Table I and Figures I and 2). B.O. I and B.O. Ill, which, according to El-Rafey est al. (1944), include phospho­ lipids showed very little oxidation intensity in all systems. B.O. II which is devoid of phospholipids showed higher oxidation intensity in phosphate buffer than in either water or milk serum systems. El-Rafey jit ail. (1944) showed that the improved keeping quality of B.O. I and B.O„ III is due to the denaturing effect of heat on the phospholipidprotein complex which passes from the non-oil phase to the oil phase as a result of the specific processing treatments. These workers also found the concentration of reducing substance (mostly -SH bearing compounds) to be much higher in oils made by the boiling off process than by the low heat treatment. The low oxidation intensity of the butter oil preparations in the presence of copper ions alone or copper ions plus ascorbic acid in com­ parison to the much higher rates obtained with the fat globule membrane and dialyzed cream is in support of the conclusion that the fat globule membrane is the site of oxidized flavor development in aqueous media. -40- The Role of gulfhydryl Groups (L-cysteine), Whole Casein and Kappa Casein Compounds containing S-H links are important in oxygen transport in lipid oxidation in biological systems, but the way in which they catalyze lipid oxidation is not fully understood. The role of casein and K-casein may be dependent on their cysteine content. Beeby (1964) has shown that freshly prepared Krcasein contains cysteine and not cystine as was former­ ly believed. Hill (1964) concluded from his results that casein micelles contain cysteine and that it is likely that cystine is not present. (I) fractions: The effect of L-cysteine on the autoxidation of milk lipid The behavior of an increasing concentration of L-cysteine on the autoxidation of the various milk lipid fractions was dependent on both the particular lipid fraction used and the composition of the aqueous phase in which the reaction took place (Figures 8 and 9). Higher concentrations of L-cysteine were significantly pro-oxidant in the presence of FGM I and FGM II in water systems. However, the oxida­ tion intensity given by FQi II was much higher than that given by FGM I. A completely different trend was given in milk serum, where concentrations of L-cysteine from I x IO-lS l to 12 x IO- S l had a pro-oxidant effect in both the FQi preparations. A concentration of 12 x IO-^M gave an antioxidant effect, while higher concentration gave lower oxidation intensities. This behavior is probably explicable in terms of the changes taking place in L-cysteine between the reduced (GSH) and the oxidized (GSSG) forms„ The pro-oxidant properties of (-SH) groups cease when they are oxidized to disulfide (-S-S-) or sulfate (Tait and King, 1936). Like ascorbic acid, cysteine is a reducing agent which converts cupric to cuprous state. -41- _______ . FGM I --------- , FGM I I D i a l y z e d Cream (A b s o rb a n c e ) in n 11iiininmiiiiiiii. HjitltUU4 (ii1iiiJ- L - c y s t i e n e (10"^ M /1) F i g u r e No. 8 : The effect of increasing the concentration of L-cysteine in the presence of 4 ppm Cu+"** in water system on the autoxidation of milk lipid fractions. -42- •---------- • 0.4 FGM I FCM II Dialyzed Cream B .0. I B.O. II 0.3 h (Absorbance) B.O. Ill a 0.2 0.1 0 —0.05 t I Figure No. 9: i _____I_____ I_____ I----- 1— 9 12 15 18 21 L-cysteine (IO-^M/I) l 3 t 6 The effect of increasing concentration of L-cysteine in the presence of 4 ppm Cu+"*" in milk serum on the autoxidation of milk lipid fractions. -43- The following reactions were proposed by Wilkinson (1964) to explain the action of the reduced and oxidized forms of L-cysteine: GSH-. % = CS" + H+ Cu++ + GR- ■;■■■-. — A GS- + Cu+ GS- + RH + R- ^ GSH These reactions indicate that the reduced form of cysteine initiates the production of free, radicals (R) from the unsaturated fatty acids„ Oxidation with irreversible formation of (QSSG) at the pH of the milk system (near pH 7.0) may be represented by the following overall reaction: 2 CS" + 2 C u + + .... — ^r GSSG X I + 2 Cu+ Conditions favoring thg predominance of the reduced .form of L-cysteine would make L-cysteine pro-oxidant; in nature, while conditions favoring the oxidized form would make it antioxidant in nature. The re­ duced pro-oxidant form is mpst likely to be predominant in w^ter systems, probably due to the dearth of milk serum salts. In milk serum systems, as the concentration of L-cysteine increases, the concentration of the oxidized L-cysteine form (GSSG) increases gradually due to the predominance of cupric salts. %his makes L-cysteine progressively antioxidant at its higher con­ centrations , These assumptions ere supported by the findings of several investigators. Hopkins (1925) studied the oxidation of emulsified un­ saturated fatty acids and found that at pH 3-4 glutethione (GSH) catalyzed lipid oxidation, but at pH 7.4 to 7.6, the gluathione was rapidly autoxidized and lipid oxidation ceased. results using lecithin. Tait and King (1935) obtained similar They observe^ that when the oxidized form of - -.44- glutathione (GSSG) was adde4, no catalysis of lecithin oxidation occurred. The reduced form of glutathione, while strongly catalytic, was recovered by them ffom the reaction mixture apparently unaltered ^t the conclusion of oxidation. These results were latef subtantiated by Busch and Kline (1941) who used both cysteine and glutathione fpr the oxidation of phos­ pholipids. The behavior of L-cysteinp in the presence of FGM I and FQl II ■ seems to follow the atiove described pattern, although compositional differences between these two membrane preparations were apparent. All concentrations of L-cystefpe in both the weter and milk serum systems acted as a powerful antioxident with the tjhree butteroil prepara­ tions. These results agree witU those of ElTRafey £t al. (1944) who found that increased concentrations of the reduced substances in butteroils made by the "boiling-off" method gave thent very high stability against oxidation in comparison to oils prepared by the low heat treatment. Dialyzed cream, which contains both the fat glpbule membrane and the true fat in their natural condition, gave an oxidative behavior simirIar to that of FGM II in water systems and FGM I in milk serum. The oxidation intensity wag much lower than that given by the FQl material. In all probability, phis ig a reflection of the lower concentration of the FQl material present, which according to the results obtained in the present investigation, are the site for oxidized flavor development. (2) The effect of casein and Kappa casein: The behavior of whole casein and K-casein during the autqxidation of milk lipid fractions was dependent on the substrate arid the nature of fhe aqueous phase into which -45- it was dispersed (Figures 10, 11, 12, and 13). The increased concentration of whole casein exerted a significant antioxidant effect with FCM I and dialyzed cream in water systems, but behaved as a pro-oxidant with FGM II. The behavior with FCM I and dialized cream was dependent on the concentration of casein. Concentrations of 0.50-1.0% were pro-oxidant but higher concentrations were antioxidant. All three milk lipid fractions showed practically no significant oxidation in milk serum systems at all casein concentrations. . Increased concentrations of K-caseiii in water systems gave the reverse of the trend given with FGM II in water.systems. Concentrations up to 1.5% K-caseih were pro-oxid@nt, while higher concentrations were antioxidant. The autoxidation of FGM I and dialyzed cream was suppressed substantially in the presence of an increased concentration of K-casein. This was the same trend obtained with whole casein, which gave much lower oxidation intensity with these lipid fractions. The autoxidation of these three milk lipid fractions was suppressed drastically in milk serum systems and in the presence of K-casein at all concentrations„ This behavior is similar to that given by whole casein. Whole casein and K-casein, when present in a salt free system, probably act as pro-oxidant. This was suggested by El-Negoumy (1965). In the case of FGM I and dialyzed cream, this was true at lower concentra­ tions of these proteins where the ratio of protein to copper was enough to form the cuprous-protein-complex. Higher concentrations of proteins favor protein in a free form which acts as an antioxidant. In the case of FGM II, probably differences in its make up and composition counteracted -46- * FGM I (Absorbance) Dialyzed Cream Casein (7.) Figure No. 10: The effect of increasing concentration of casein in the presence of 4 ppm Cu++ in water system on the autoxidation milk lipid fractions. -47- TBA (Absorbance) Dialyzed Cream Casein (%) Figure No. 11: The effect of increasing concentration of casein in the presence of 4 ppm Cu+"*" in milk serum on the autoxidation of milk lipid fractions. —48— " niiuiti,'f Dialyzed Cream (Absorbance) Y K-casein (%) Figure No. 12; The effect of increasing concentration of K-casein in the presence of 4 ppm Cu++ in water system on the autoxidation of milk lipid fractions. 49 - TBA (Absorbance) - Dialyzed Cream K-Casein (%) Figure No. 13: The effect of increasing concentration of K-casein in the presence of 4 ppm Cu+"** in milk serum on the autoxidation of milk lipid fractions. -50- the antioxidant effect of higher casein concentration favoring the pro­ duction of the catalytic form of copper. Freiden and Alles (1958) showed that proteins act as agents for the reduction of copper ions when the latter is present at concentrations higher than I ppm. Smith and Dunkley (1962 a,b) substantiated these, results by detecting cuprous copper with I neocuproine. Lea (1936) obtained results indicating that the copper pro­ tein ratio may influence the catalytic power of copper in the presence of proteins. Using copper concentrations of 0.4 to 1.5 ppm with 4% albumin, he found that copper catalysis ceases at low ratios of copper to protein. The reason that higher concentrations of K-casein were antioxidant in water systems with FGM II, while higher concentrations of whole casein were pro-oxidant is difficult to explain, The explanation probably lies within the biochemical nature of both FQd II and K-casein. It may be that K-casein, which has been reported by Beeby (1964) to be the cysteine bearing casein component, releases its -SH groups by interactions with FQd II. These released sulfhydryl groups may then increase the anti' I oxidarit properties of K-casein. ' ' ' ' Tarassuk et al. (1959) and Koops et al. ■ (1959) found that fishy flavor (an intensified oxidized flavor) in butter can be inhibited by adding heated skim milk or whey to washed cream from which the buttier was made. They attributed this effect to the released -SH groups, since this inhibiting effect was removed by blocking the -SH groups with N-methylmaleiamide. Forester and Sommer (1961) also attributed the protective action of milk serum proteins against oxidized flavor devel­ opment to their releasable -SH groups. According to El-Negoumy (1965), the strong antioxidant behavior -51- of both whole casein and K-casein in milk serum systems is due to the complexing of the copper by the anions of milk salts in the serum. He suggested that this spares the protein, which then exerts its antioxidant properties, especially at its higher concentrations. The Effect of Antioxidants Although the present United States standards do not permit anti­ oxidants in dairy products and hence the question of their effectiveness L is one of theoretical interest, they are of practical interest in other countries where their use is permitted. (I) The effect of Nordihydroguaiaretic acid (NDGA); the anti­ oxidant properties of NDGA varied according to the milk lipid fraction used and the composition of the equeous phase ip which they were dispersed. Comparison of the data in Table I and Figures 14 and 15 indicate that NDGA showed strong antioxidant properties in both water and milk serum system with FQeI I, F(34 II and dialyzed cream. NDGA is a more effective anti­ oxidant with FGM I and FGM II than with dialyzed cream. Shelton (1959) described four mechanisms by which antioxidants function as a chain stopper for the free radical chain mechanism of lipid oxidation. apply to phenolic antioxidants like NDGA: Two of these (I) addition of the lipid to the aromatic ring of NDGA or (2) formation of a complex between the lipid and the aromatic ring of the antioxidant. The reason that NDGA did not exert its antioxidant properties with the same degree of effectiveness in FGM I and FGM II is probably due to configurational differences between the two. In other words, one may be able to form complexes or add to the NDGA more effectively than the other. ->52r* The strong antioxidant properties of WDGA in a milk serum system probably arise from the synergistic effects of some of the salts present in milk serum. According to Stuckey (1962), the effect of these syner­ gists is probably due to hydrogen radical or electron donation„ The polybasic salts, especially the citrates and phosphates are the most pre­ dominant syngergists in milk serum. According to Badings (1961) and. Jenness and Patton (1959), the synergistic effects of these polyhasic salts may be due to their seques­ tering effect on metallic ions, According to Privett and Quakenbush (1954), these synergists exert their greatest influence at relatively low levels. (2) The effect of o(-tocopherol; Alpha-tocopherol (vitamin E) is a well known antioxidant in nature. Horwitt (1961) presented lines of evidence that the main function of o(-tocopherol is that of a lipid anti­ oxidant and pointed to the important relationship of this antioxidant and polyunsaturated fatty acids in the diet. Alpha-tocoperol behaved as an antioxidant with FGM I, FGM II and dialyzed pream in milk serum systems (Figures 16 and 17). According to Tappel (1962), the chemical basis for vitamin E functioning as an anti­ oxidant lies in its reaction with the free radical intermediates of lipid peroxidation and lipid peroxides through which lipid peroxidation is in­ hibited. However, higher concentration of ©(-tocopherol were less effective with FQl II in milk serum systems. It is suggested that FQl II may destroy the antioxidant properties of o(-tocopherol in the presence of milk serum salts by oxidizing of-tocopherol to of-tocopherylquinone. According to Tappel -53- (1962), o(-tocopherylquinone is the major product of oxidation during autoxidation of lipids. tocopherol -54- 0.3 ,__________ , FGM I FQl II TBA (Absorbance) ,__________« 0.1 Figure No. 14: 0.2 0.3 0.4 0.5 NDGA (7o) 0.6 0.7 0.8 The effect of NDGA in the presence of 4 ppm Cu h " in water system on the autoxidation of milk lipid fractions. - 55 - 0.2 r (Absorbance) 0. 1 ___________ . FGM I ___________ _ FGM II ^ ^.11D11i in n 11/i 11ji/. D ia ly z e d Cream 0 m H - 0.1 ■ 0.1 Figure No. 15: 0.2 0.3 0.4 0.5 NDGA (%) 0.6 0.7 0.8 The effect of NGDA in the presence of 4 ppm Cu h " in milk serum system on the autoxidation of milk lipid fractions. -56- TBA (Absorbance) Dialyzed Cream .^L L L L L h 111 i I I I I Iiin ^ o^-tocopherol (in acetate, ug/lOOml) Figure No. 16: The effect of the antioxdant property of c < -tocopherol in water system on the autoxidation of milk lipid fractions. -57- (Absorbance) 0.6 Dialyzed Cream tocopherol (in acetate; ug/lOOml) Figure No. 17: The effect of the antioxidant property of ^-tocopherol in milk serum system on the autoxidation of milk lipid fractions. SUMMARY Six different fractions of milk lipids were subjected to autoxidation in model systems composed of either distilled water.or milk serum, and occasionally pH 6.60 phosphate buffer. All the model systems contained 2% lipid material, Autoxidation was allowed to proceed for 48 hours at 3-5°C. The rates and intensities of oxidation were measured in terms of the absorbance of the 2-thiobarbituric acid test (TEA). The effects of the following factors were investigated: (I) Presence of copper ions alone, ascorbic acid alorte, or copper plus ascorbic acid. (2) Presence of sulfhydryl compounds (L-cysteine), whole casein, and K-casein. (3) Presence of synthetic antioxidant (NDGA) and naturally occurring antioxidant ( o(-tocopherol). The following is a summary of the most important findings: (1) The fat globule membrane material is the most likely site for oxidized flavor development in aqueous dairy products. This is demonstrat­ ed by the fact that fat globule membrane material showed a higher oxidai ' tion intensity and rate in comparison with butteroil preparations. (2) Copper alone and ascorbic acid alone catalyzed oxidized flavor development in the fat globule membrane and the intact fat globules at rates and intensity dependent on the nature of the aqueous, medium used. (3) The highest oxidation intensifies took place in the presence of copper plus ascorbic acid. Theoretically, during the storage period of dairy products, ascorbic acid is oxidized and in the course of doing so, it reduces cupric ions to the catalytic-active cuprous state. (4) The catalytic effect of cuprous ions in initiating autoxida­ tion is dependent on the kind and amount of anions which complex with it. -59- Cuprous phosphate is a much more potent catalyst than cuprous citrate formed in milk serum. (5) Copper ions (4 ppm) from various salts gave variable oxidation rates and intensities in similar systems, indicating varied catalytic power of the different anions complexing with cuprous ions. (6) True fat, in the form of butteroils, gave the lowest oxida­ tion intensities, indicating that it is not the primary site of oxidized flavor development in aqueous dairy products. The boiling-off method is suggested as the best way to prepare pure milk fat of the best keeping qualities. (7) The fat globule membrane from butter serum (FGM II) generally gave a much higher oxidation intensity in the presence of copper ions and ascorbic acid in.comparison to that from buttermilk (FGM I). This is indicative of significant compositional differences between the two mem­ brane fractions. This difference could either be due to ,different proteins in the membrane preparations or to differences in the concentration of polyunsaturated fatty acids and amounts of natural copper ions. (8) L-cysteine behaved either as a pro-oxidant, or as an anti­ oxidant , depending on the milk lipid fraction used and on the nature of the aqueous medium in which the lipid was dispersed. In water systems, it promoted the autoxidation of the fat globule membrane material, while it inhibited the oxidation of the butteroil prepartions. In milk serum, L-cysteine acted as an antioxidant at its lower concentrations with both FGfcl I and FGM II and as a pro-oxidant at its higher concentrations. In ttie aqueous phase of natural milk serum, salt's are probably the factor to -60- influence the oxidation of (-SE) groups to disulfide form and change its pro-oxidant behavior. (9) Increasing the concentration of whole casein inhibited the oxidation of FGM I and dialyzed cream in water systems but promoted the oxidation of FQl II. The autoxidation of these lipid fractions was com- J pletely suppressed by casein in milk serum systems. Concentrations of 0.5 to 1.5% of K-casein acted as pro-oxidant with FQl II and dialyzed cream in water systems but in higher concentrations acted as an anti­ oxidant. K-casein was antioxidant at all concentrations with FQl I . In milk serum systems, all concentrations of K-casein were strongly anti­ oxidant. Milk proteins exert their antioxidant properties at higher pro­ tein concentrations and lower copper concentrations. Their antioxidant properties are enhanced by the synergistic effect of milk serum salts. (10) Norhydroguaiaretic acid (NDGA) showed a strong antioxidant effect on the milk fat globule membrane. The effectiveness was depressed at concentrations higher than 0.5% and was enhanced by the synergistic effect of milk serum salts. The naturally occurring antioxidant o(-tocopherol behaved as a more effective antioxidant at its higher concentrations by intercepting and interacting with the free radicals required for initiating autoxidation of lipids. APPENDIX - 62TABLE NO. II The Effect of Increasing Concentration of Ascorbic Acid In The Absence of Cu++ On The Autoxidation Of Milk Lipid Fractions (Absorbance) Reaction Medium Milk Lipid Fractions Water System . Absorbic Acid (mg/lOOml) 0.5 I 4 8 12 16 20 24 FGM I 0.014 0.096 0.365 0.555 1.155 1.005 0.995 0.105 FGM II 0.347 0.482 1:457 1.907 > 2 .00* > > 2 .00* > > 2 .00* > 2 .00* D. Cr. 0.003 0.037 0.109 0.110 0.089 0.099 0.084 0.119 B.O. I 0.015 0.017 0.017 0.024 0.028 0.043 0.053 0.060 B.O. II 0.006 0.012 0.016 0.016 0.056 0.022 0.022 0.040 B.O. Ill 0.034 0.034 0.046 0.047 0.038 .0.045 0.044 0.043 Milk Serum System FQl I 0.055 0.052 0.019 0.032 0.237 0.092 0.089 0.137 FGM II 0.428 0.518 1.078 0.328 1.218 1.218 1.168 1.118 D. Cr. 0.055 0.050 0.040 0.048 0.042 0.048 0.055 0.050 B.O. I 0.010 0.014 0.007 0.022 0.018 0.021 0.022 0.015 B.O. II 0.009 0.013 0.014 0.035 0.017 0.014 0.032 0.028 B.O. Ill 0.009 0.009 0.008 0.002 0.009 0.004 0.005 0.006 value larger than 2 -63TABLE NO. Ill The Effect of Increasing Concentration of Ascorbic Acid In The Presence Of Cu*"*" On The Autoxidation Of Milk Lipid Fractions (Absorbance) Reaction Medium Milk Lipid Water System Fractions _____________________________ _ Absorbic Acid (mg/100ml) 0.5 I 4 8 12 16 20 24 Fm i 0.255 0.285 0.360 0.395 0.465 0.565 0.565 0.605 FGM II 0.367 0.427 0.857 1.007 0.907 0.687 0.827 0.797 D. Cr. 0.032 0.043 0.143 0.195 0.252 0.229 0.292 0.402 B.O. I 0.017 0.023 0.035 0.059 0.069 0.105 0.097 0.080 B .0. II 0.000 0.000 0.030 0.030 0.033 0.042 0.057 0.060 B.O. Ill 0.004 0.007 0.038 0.032 0.057 0.054 0.060 0.060 Milk Serum System Fm i 0.117 0.117 0.207 0.337 0.272 0.337 0.307 0.278 F m ii 0.138 0.158 0.238 0.568 0.348 0.388 0.323 0.218 D. Cr. 0.860 0.780 0.122 0.116 0.146 0.139 0.121 0.122 B.O. I 0.008 0.014 0.040 0.098 0.117 0.108 0.127 0.149 B.O. II 0.009 0.010 0.044 0.033 . 0.067 0.075 0.067 0.050 B.O. Ill 0.000 0.003 0.032 0.055 0.084 0.095 0.075 . 0.079 -64TABLE NO. IV The Effect of Copper Ion From Various Sources In The Presence Of Ascorbic Acid in Water and Milk Serum Systems On The Autoxidation Of Milk Lipid Fractions (Absorbance) Reaction Medium Copper Salts ppm Distilled Water 0.5 I 4 8 12 16 20 24 Cu(CH3COO)2 0.059 0.074 0.124 0.159 0.154 0.174 0.154 0.124 CuCO3 0.094 0.104 0.139 0.134 0.204 0.194 0.184 0.149 0.124 0.094 0.199 0.249 0.249 0.254 0.204 0.139 Cu(NO3)2 0.084 0.074 0.134 0.114 0.214 0.204 0.194 0.134 CuSO^ 0.032 0.043 0.134 0.195 0.252 0.229 0.292 0.402 Milk Serum System Cu(CH3COO)2 0.120 0.140 0.100 0.100 0.070 0.075 0.080 0.075 CuCO3 0.135 0.125 0.105 0.085 0.085 0.080 0.075 0.070 CuCl2 0.120 0.090 0.080 0.100 0.090 0.075 0.100 0.075 Cu(NO3)2 0.075 0.080 0.075 0.085 0.085 0.080 0.090 0.100 CUSO4 0.086 0.078 0.122 0.116 0.046 0.139 0.121 ,0.122 -65TABLE NO. V The Effect Of Varying Fat Content (from Dialyzed Cream) In The Presence of 4 ppm Cu*-1" And An Increased Concentration of Ascorbic Acid In Milk (Serum System On the Autoxidation Of Milk Lipid Fractions (Absorbance) Ascorbic Acid mg/iOOml 0.5 1.0 4 8 12 16 20 24 27. fat 0.055 0.050 0.040 0.048 0.042 0.048 0.055 0.050 4% fat 0.150 0.120 0.090 0.090 0.075 0.070 0.070 0.060 6% fat; 0.150 0.130: 0.135 0.170 0.170 0 .155 0.121 Q-ISOl 87. fat 0.285 0.320 0.370 0.430 0.440 0,450 0.410 0.350 107. fat 0.450 0.460 0.520 0.430 0.220 0.210 0.120 0.100 127. fat 0.350 0.330 0.460 0.545 0.760 0.950 0.650 0.700 -6 6 - TABLE NO, VI The Effect of Increasing Concentration of L-cysteine In The Presence Of Copper Ion And 2% Of Lipid On The Autoxidation Of Milk Lipid Fractions (Absorbance) Reaction Medium Milk Lipid Fractions Water System L-cysteine 10"^ M/1 I 3 6 9 12 15 18 21 FGM I 0.145 0.146 0.146 0.152 0.345 0.350 0.351 0.350 FQl II 0.267 0.287 0.377 0.597 0.537 0.667 0.877 0.907 D, Cr, 0.065 0.054 0.048 0.045 0.045 0.044 0.054 0.058 B,0, I -0.003 -0.003 0.007 0.013 0.008 0.005 0.007 0.004 B,0. II +0.004 -0,005 -0.073 0.006 +0.003 -0.004 0.001 -0.602 B,0, III -0.002 -0.002 0.010 0.008 0.007 0.005 0,007 0.008 Milk Serum System FQl I 0.010 0.280 0.280 0.310 6.400 0.270 0.140 0.200 FQl II 0.030 0.068 0.098 0.183 0.093 -0.012 0.048 0.138 D. Cr. 0.075 0.064 0.063 0.070 , 0.065 0.050 0.055 0:052 B.O. I 0,002 -0.003 0.000 0.003 0.003 -0.003 0.007 0.007 B.O. II 0.011 0.008 0.011 0.009 0.011 0.505 0.015 0.005 B.O. Ill 0.003 -0.001 -0.005 0.007 -0.003 -0.007 -0.001 -0.001 . TABLE NO. VII The Effect Of Increasing Concentration Of Casein In Presence Of Copper Ion On The Autoxidation Of Milk Lipid Fractions (Absorbance) Reaction Medium Milk Lipid Fractions Milk Serum System Water Systein 2.0 Casein % 2.5 0.5 1.0 FGM I 0.045 0.065 -0.030 -0.040 -0.050 -0.098 -0.198 -0.092 -0.118 -0.198 FGM II 0.147 0.117 0.232 0.227 0.282 -0.102 -0.107 -0.082 -0.018 -0.032 D. Cr. 0.124 0.124 0.094 0,084 0.084 -0.117 -0.126 -0.119 -0.127 -0.197 1.5 0.5 1.0 1.5 2.0 2.5 67- ;! TABLE NO. VIII The Effect Of Increasing Concentration of Kappa Casein In The Presence Of Copper Ion On The Autoxidation Of Milk Lipid Fractions (Absorbance) ____ _____ Milk Lipid Fractions Water System O 5 - i Q . Reaction Medium_________________________ i.5 ____________________________Milk Serum System Kappa Casein %______ ___________ _______ 2,0 2.5 0,5 1.0 1.5 2.0 2.5 F(M I -0.001 0.005 =0.059 -0.030 -0.025 -0.078 -0.023 -0.063 -0.113 =0.133 FGM II 0.137 0.127 0.177 0.042 0.027 0.008 -0.087 -0.072 -0.132 -0.152 D. Cr. 0.022 0.052 -0.060 -0.100 -0.110 0.038 -0.022 -0.162 -0.187 -0.147 “68- TABI4E NO. IX The Effect of NDGA In The Presence Of Copper Ion On The Autoxidation of Milk Lipid Fractions (Absorbance) Reaction Medium Milk Lipid Fractions Water System 0.1 0.2 0.3 FOl I 0.110 0.080 0.090 FCM II 0.207 0.127 D. Cr. 0.004 0.009 NDGA % 0.4 0.5 0.6 0.7 0.8 0.095 0.065 0.045 0.000 0.005 0.092 0.117 0.072 0.137 0.147 0.147 0.014 0.019 I 0.014 0.029 0.029 0.029 Milk Serum System FOl I -0.013 -0.930 -0.113 -0.123 -0.043 0.027 0.049 0.077 FOl II -0.022 0.012 -0.052 -0.042 -0.022 0.012 -0.042 0.012 D. Cr. 0.050 0.040 0.040 0.030 0.040 0.055 0.045 0.045 69- TABLE NO. X The Effect Of The Antioxident Property o f t o c o p h e r o l On The Autoxidation Of Milk Lipid Fractions (Absorbance) Rea1Ctiori Medium Milk Lipid Fractions _ Water System I • ef-tocopherol mg/IOOml 80 100 40 60 FGM I ' 0.565 0.405 0.505 0.445 Fm ii 0.237 0.177 0.167 D„ Cr. 0.093 0.123 0.088 120 140 160 0.235 0.105 0.145 0.065 0.177 0.167 0.167 0.167 0.117 0.133 0.028 0.023 0.021 0.021 Milk Serum System FGM I 0.337 0.357 0.227 0.293 0.262 0.157 0.117 0.077 Fm ii 0.040 0.060 0.148 0.138 0.138 0.168 0.158 0.168 D . Cr. 0.080 0.080 0.080 0.095 0.080 0.045 0.038 0.038 LITERATURE CITED Badings$ H= T» 1961. Netherlands Milk and Dairy J. 14;215 = (Cited from Wilkinson, R. A. 1964. Theories of the mechanisms of oxidized flavor development in dairy products, Div. of Dairy Res., Comm. Sci. and Ind. Res. Org. Melbourne). Bassette, R. and M. Keeney. 1960. Identification of some volatile carbonyl compounds from nonfat dry milk. J. Dairy Sci. 43:1744. Beeby, R. 1964. The presence of sulfhydryl groups in K-casein. Biophys. Acta. 82:418. Biochem« Biggs, D. A. and L. R. Bryout,. 1953. The thiobarbituric acid test for butter fat oxidation. Can. J. Techn. 31:138. Holland, J. L. and G. Gee. 1946. Kinetic studies in the chemistry of rubber and related materials. III. Thermochemistry and mechanisms of olefin oxidation. Trans. Farady Soc. 42:244. Brunner, J. R., C . W. Duncan and G. M. Trout. 1953a. The fat globule membrane of non-homogenized and homogenized milk. I. The isolation and amino acid composition of the ,fat membrane proteins. Food Res. 18, 5:454. Brunner, J. R., H. H, Lillevik, 6 . M. Trout and C. W. Duncan. 1953b. The fat globule membrdne of non-homogenized and homogenized milk. II. Differences in the electrophoretic patterns of the fat-membrane proteins. Food Res. 18, 5:463. Brunner, J. R. 1962. Protein-lipid interactions and their relation to the physical-chemical stability of concentrated milk. A Review, J. Dairy Sci. 45:943. Corbelt, W. J. and P. H. Tracy. 1941. Experiments on the use of certain antioxidants for control of oxidized flavor in dairy products. . Food Res. 6:445. Day, E. A. and D. A. Lillard. 1960. Autoxidation of milk lipids. I. identification of volatile monocarbonyl compounds from autoxidation of milk fat. J. Dairy Sci. 43:585. Dunkley, W. L. and W. G. Jennings. 1951. A procedure for application of the thiobarbituric acid test to milk. J. Dairy Sci. 34:1064. Edwin, E. E., J. Bunyan, A. T,. Diplock and J. Green. 1961. Role of tocopherol, selemium and antioxidant in rat. Nature. 189:747. El-Negoumy, A. M., D, M. Miles and E. G. Hammond. 1961. Partial character­ ization of the flavors of oxidized butteroil. J. Dairy Sci. 44:1047. -71- El-Negoumy, A. M . , M. S, de Puchal and E« G. Hammond. 1962a. Relation of linoleate and linolenate to the flavors of autoxidised milk fat. J. Dairy Sci. 45:311. El-Negoumy, A. M . s E. G. Hammond. 1962b. Effect of Antioxidants on the flavor of cold storage butter. J. Dairy Sci. 45:848. El-tNegoumy, A. M. 1965. Relation of composition of the aqueous phase to oxidized flavor development by dialyzed globular milk fat. J. Dairy gci. 48:1406. El-Rafey, M. S., G. A. Richardson and J. L. Henderson. 1944. The effect of the manufacture qf butteroil on its keeping quality. J. Dairy Sci. 27:807. Farmer E. H. and A. Sundralingam. 1943a. The course of autoxidative reaction in pqlyisoprenes and allied compounds. Part I. The structure and relative tendencies of the peroxides of simple olefins. J. Chem. Soc. 1943:121. Farmer, E. H. and D. A. Sutton. 1943b. The course of antioxidation reactions in polyisoprenes and allied compounds. Part III. The isolation and constitution ojE photochemicalIy formed methyl oleate peroxides. J. Chem, Soc. 1943:119. Filtman, R. and E. Freiden. 1957, Cuprous ion formation in cupric ion catalyzed reactions. J. Am. Chem. Soc. .79:5193, Forss, D. A., E. G. Pont and W. Stark. 1955.. The volatile compounds associated with oxidized flavor in skim milk. J. Dairy Sci, 22:91. Forss, D. A., E. A. Punstone and W. Stark. 1960a. Fishy flavor in dairy products. II. The volatile compounds associated with fishy flavor in putter fat. J. Dairy Res. 27:211. Forss, D. A., E. A. Dunstone and W. Stark. 1960b. Fishy flavor in dairy products. III. The volatile compounds associated with fishy flavor in washed cream. J. Dairy Res. 27:373. Forss, D. A. E. A. Punstone and W» Stark. 1960c. The volatile compounds associated with tallowy and painty flavor in butteroil. J, Dairy Res. 27:381. F o r ste r , T. L. and H. H. Sommer., 1951. ,Manganese, trypsin, milk proteins and the susceptibility of milk to oxidized flavor development. Dairy Sci. 34:992. J. -72- Freiden1 E» 1958. Colorimetric measurement of cuprous ion formation to detect certain reducing agents as illustrated with cysteine, gluta­ thione and serum albumin. Biochem. Biophys. Acta. 2.7:414. Freiden1 E. and J. Alles. 1958. Subtle interaction of cupric ion with nucleic acids and components. J. Biol. Chem. 230:797. French, C. M. and E. R. Monks. 1961. The conductance of ferric and cupric stearates in aliphatic hydrocarbons. J. Chem. Soc. 466: 468 p. Greenbank1 G. R. 1940. Variation in the oxidation-reduction potential as a cause for the oxidized flavor in milk. J. Dairy Sci. 23:725. Hammond, E. G. and F. D. Hill. 1964. The oxidized-metallic and grassy flavor components of autoxidized milk fat. J. Am. Oil Chem. Soc. 41:180. Harrison, D„ C. 1924. The catalytic action of traces of iron on the oxidation of cysteine and glutathione. Biochem. J. 18:1009. Harwalker1 V. R. and I. R. Brunner. 1965. Effect of dissociating agents on physical properties of fat, globule membrane fractions. J. Dairy Sci. 48:1139. Herald, C. T. and J. R. Brunner. 1957a. The fat-globule membrane of normal cow’s milk. I. The isolation and characteristics of two membrane protein fractions. J. Dairy Sci. 40:948. Herald, C. T., J. R. Brunner and S.,T. Bass. 1957b. A speetrographic analysis of two protein fractions of the fat-globule membrane of normal cow’s milk. J. Dairy Sci. 40:446. Hilditch, T. P. 1956. The chemical constitution of natural fats, John Wiley and Sons, Inc. 134-135 p. Hill, R. L. 1964. Constancy of amino acid composition of cow’s milk protein under changing rations. J. Dairy Sci. 47:1417. Holmes, A. D. 1952. Res. 17:367. Ascorbic acid in tocopherol-enriched milk. Food Holmes, A. D. 1953. Value of nitrogen for stabilizing reduced ascorbic acid in milk. Food Tech. 7, 3:136. Hopkins, Fi G. 1925. Glutathionine, its influence in the oxidation of fats and proteins. Biochem. J. 19:787. -7 3 - Horwitts Mo Ko 19610 Vitamin E in human nutrition review, Borden Rev, of Nutr. Res. 22si™17, Hunzikers 0« F. 7:484. 1924« Facts about carbonated butter, an interpretive * J, Dairy Sci, Jacksons R. H„ and J, R, Brunner. 1960. Characterization of protein fractions from the fat plasma interface, J. Dairy Sci. 43:912. Jennesss R. and S. Patton. 1959. Principle of Dairy Chemistry. Wiley and Sons, Inc. 375:376 p. John : Jennings, W. G., W. L„ Dunkley and H. G. Berber. 1955. Studies of certain red pigments formed from 2-thiobarbituric acid. Food Res. 20:13. Keeney, M. and F. J. Doan. 1951a. Studies on oxidized milk fat. I. Observation on the chemical properties of the volatile flavor material from oxidized milk fat. J. Dairy Sci. 34:713. Keeney, M. and F. J. Doan. 1951b. Studies on oxidized milk fat. II. Preparation of 2, 4 - dinitrophenyIhydrazones from the volatile materials from oxidized milk fat. J. Dairy Sci. 34:719. Keeney M. and F. J. Doan. 1951c. Studies on oxidized milk fat. III. Chemical and organoleptic properties of volatile material obtained by fractionation with various solvents and Girard9S reagent. J. Dairy Sci. 34:728. Kelley, G. G. and B. M. Watts. 1957. Effect of copper chelating agents on the pro-oxidant activity of ascorbic acid with unsaturated fats. Food Res. 22:308. King, N. 1954. Milk fat globule and some associated phenomena. by Commonwealth Agricultural Bureau, Royal Bucks, England. Published King, R. I. and W, L. Dunkley. 1959. Relation of natural copper in milk to incidence of spontaneous oxidized flavor. J. Dairy Sci. .42:420. King, R, L., 1962. Oxidation of milk fat globule membrane material. I. Thiobarbituric acid reaction as a measure of oxidized flavor in milk and. model systems. J. Dairy Sci. 45:1165. King, R. L. 1963. Oxidation of milk fat globule membrane material. II. Relation of ascorbic acid and membrane concentration. J. Dairy Sci. 46:267. -74- Koops, J., N. P. Tarassuk and J. W. Pette. 1959. Netherlands Milk Dairy J. 13:279, Cited from Wilkinson, R. A. 1964. Theories of the mechanism of oxidized flavor development in dairy products, Div. of Dairy Res. Comm. Scie and Ind. Res. Org. Melbourne. Krukovsky, V. N., D. A. Theokas, F. Whiting and E. S. Guthrie. 1949a. The effects of norhydroguaiaretic acid, salt and temperature of storage on the stability of fat and fat-soluble vitamins in cream and butter. J. Dairy Sci6 32:679. Krukovsky, V. N., D. A. Theokas and F. A. Whiting. 1949b. dant properties of nordihydroguairetic acid in cream. 32:695. The antioxi­ J. Dairy Sci. Lea, C. H. 1936. Antioxidants and the preservation of edible fats. Soc. Chem. and Ind. 55:293T. Lea, C. H, 1962. Symposium on Foods: Lipids and their oxidation. AVI Publishing Co., Inc., Westport, Conn. 3 p. . Liversedge, M. and H. I. Kohn. 82:2921. 1944. J. The J. Pharmacol, and Exp. Therap. Lundberg, W. O., W. B. Dockstade and H. G. Halvorson. 1947a. The kinetics of oxidation of several antioxidants in oxidized fats. J. Am. Oil Chem. Soc. 24:89. Matill, H. A. 1931. Chem. 90:141. Antioxidants and the autoxidation of fats. J. Biol. McDowell, A. K. R. 1955. The effect of salt and of autoxidants on the keeping quality of butter. J. Dairy Res. 22:349. Meyerhof, 0. 1918. Pflug'. Arch. ges. Physiol., 170:428. Cited from Wilkinson, R. A. 1964. Theories of the mechanisms of oxidized flavor development in dairy products, Div. of Dairy Res. Comm. Sci. and Ind. Res. Org. Melbourne. Meyerhof, 0. 1923. Pflug. Arch. ges. Physiol., 199:531. Cited from Wilkinson, R. A. 1964. Theories of the mechanisms of oxidized flavor development in dairy products, Div1. of Dairy Res. Comm. Sci. and Ind. Res. Org. Melbourne. Morton, R. K. 1954. J. 57:231. The lipoprotein particles in cow's milk. Biochem. Nelson, S. M. and K. C. Pink.. 1954. Solutions of metal soaps in organic solvents. IV. Direct current conductivity in solutions of some metal oleate in toluene. J. Chem. Soc. 4412:4417 p. "75Olson, Fo Co and W» C. Brown, 1942. Oxidized flavor in milk. XI. Ascor­ bic acid, glutathione and hydrogen peroxides as mechanism for the production of oxidized flavor. J. Dairy Sci. 25:1027. Palmer, L. S. and E. Samuelsson. 1924. The nature of the substance absorbed on the surface of the fat globule in cow 9S milk. Proc. Soc. Expt. Biol. Med. 21:537. Parks, 0. W. and S. Patton. 1961. Volatile carbonyl compounds in stored dry whole milk. J. Dairy Sci. 44:1. Patton, S. and G. W. Kurtz. 1951. detecting milk fat oxidation. 2-Thiobarbituric acid as reagent for J. Dairy Sci. 34:669. Patton. S. 1962. Dairy products. Cited from symposium oil foods: and their oxidation. The AVI,Publishing Co., Inc., Westport, Conn. 190 p. Lipids Privett, 0= S. and F. W. Quakenbach. 1954. Effects of antioxidants on the thermal decomposition of fat peroxides in vacuo. J. Oil Am. Chem. Soe. 31:281. Ramachandran, K. S. and M= L. R. Whitney. 1960. Isolation and characteri­ zation of a membrane protein fraction from the fat-globule membrane of raw milk. J. Dairy Sci. 43:854. Richardson, T. and P. L. Guss. 1965. Lipids and metals in fat globule membrane fractions. J. Dairy Sci. 48:523. Rogers, L. A. and C. E. Grey. 1909. Bull. Bur. An. Ind., B. S. Dept. Agri., 114. Cited from Wilkinson, R. A. 1964, Theories of the mechanism of oxidized flavor development in dairy products. Div. of Dairy Res., Comm. Sci. and Ind. Res. Org. Melbourne. Rusch, H. P . -and B. E. Kline. 1941. Cancer Res. 1:465. Cited from Wilkinson, R. A. 1964. Theories of the mechanisms of oxidized flavor development in dairy products. Div. of Dairy Res. Comm. Sci. and Xnd. Res. Org. Melbourne. Shelton, J. R. I9$9. Mechanism of antioxidant action in the stabilisation of hydrocarbon systems.' J. AppI. Pol. Sci. 2, 345:358. Sidwell, C. G., H. Salwin, M. Benca and J. M. Mitchell. thiobarbiturie acid as a measure of fat oxidatipn. Soc. 31:603. 1954. The use of J. Am. Oil Chem. Sinnhuber, R. 0. and T. C. Yu. 1958. 2-Thiobarbiturie acid method for the measurement of rancidity in fishery products, II. The quantitative determination of malonaldehyde. Food Tech. 12:9. -76- Smith 9 L. M o s E„ N. Frankel9 W. Haab and E. L. Jack. 1958, Effect of phospholipids and unsaponificable matter on oxidative stability of milk fat. J. Dairy Sci. 41:472„ Smith9 G. J. and W. L« Dunkley. 1962a. Pro-oxidants in spontaneous development of oxidized flavor in milk. J. Dairy Sci. 45:170. Smith9 G. J. and W. L. Dunkley. 1962b. Ascorbic acid oxidation and lipid peroxidation in milk. J. Fd. Sci. 27:127. Smith9 L. M. and R. R. Lowry. 1962. Fatty acid composition of the phos­ pholipids and other lipids in milk. J. Dairy Sci, 45:581. Sommer9 H. H. 1951. Fat emulsion in milk from a chemical standpoint. Milk Dealer. 41, 1:58. Stark9 W. and D. A. Forss. 1962. A compound responsible for metallic flavor in dairy products. I. Isolation and identification. J. Dairy Res. 29:173. Storch9 V. 1897. 22:197. On structure of 11Fat Globules'* in cow's milk. Analyst. Stuckey, B. N. 1962. Antioxidants. ,Cited from Symposium on Foods; Lipids and their oxidation (Editor; H. W. Schultz, E, A. ,Day"and R.. 0. Sinnhuber). The AVI Publishing Gompany9 Inc., Westport9 Conn. 139 p. Stull, J. W., E. 0. Herreid and P„ H. Tracy. 1947. A study of the'use of nordihydroguaiaretie acid in the storage of frozen sweet cream. J, Dairy Sci. 30:541. Stull, J. W., E. 0. Herreid and P. H. Tracy. 1948a. A study of the use of the antioxidant nordihydroguaiaretic acid in dairy products. I. Its antioxygenie properties in milk. J. Dairy Sci. 31:449. Stull9 J. W . , E. 0. Herreid and P. H. Tracy. 1948b. A study of the use of the antioxidant nordihydroguaiaretic acid in dairy products. II. Its antioxygenie properties in unsweetened frozen cream. J. Dairy. Sci. 31:1024. Stull, J. W., E. 0. Herreid and P. H. Tracy. 1949. A study of the use of the antioxidant nordihydroguaiaretic acid in dairy products. III. Its antioxygenie properties in sweetened frozen cream. J. Dairy Sci. 32:301. -77- \ ' Stull, J. W», E 0 0« Herreid and P 0 H» Tracy, ,1951a. A study of tfie use of nordihydroguaiaretic acid in dairy products. IV. Its axitioxygenic properties in spray-dried milk and ice cream mix with and without added synergists. J. Dairy Sci. 34;80. Stull, J. W., E. 0. Herreid and P. H. Tracy. 1951b. A study of.the. effect of nordihydroguaiaretic acid on the oxygen absorption of the phos­ pholipids fraction of milk. I. The effect of concentration of anti­ oxidant . J. Dairy Sci. 34:181. Swanson, A. .M. and H. H. Sommer. 1940. Oxidized flavor in milk: I. Effect of the development of oxidized flavor on the iodine number of the phospholipid fraction of milk. J. Dairy Sci. 23:201. Tait, H. and E. J. King. 1936. The oxidation of lecithin and other fatty substance in the presence of glutathione. Biochem. J 0 30:285. Tappel, A. L. 1962. Vitamin E and selenium in the in vivo lipid peroxida­ tion.' .Cited from,Symposium on Foods: Lipids and their oxidation. The AVI Publishing Co.,Inc., Westport, Conn. 367 p„ Tarassuk, N. P., J. Koops and J. W. Pette. 1959. Netherlands Milk Dairy J. 13:258. Cited from Wilkinson, R. A. 1964. Theories of the mechanism of oxidized flavor development in dairy products. Internal Report 4, Div. of Dairy Res., Comm. Soci. and Ind. Res. Org. 'Melbourne. Tarassuk, N» P. and J. Koops. 1960. Inhibition of oxidized flavor in homogenized milk as related to the concentration of copper and phos­ pholipid per unit of fat globule surface. J. Dairy Sci. 43:93. Tharp, B. W. and S. Patton. 1960. The coconut-Iike flavor defect of milk fat. IV. Demonstration of ^ -dodecalaetone in the steam distillation of fat. J. Dairy Sci. 43:475. Thurston, L. M., W. C. Brown and P. B. Dustman. 1935.. A study of the relation of materials absorbed on the fat globules to the richness of flavor of milk and certain milk products. J. Dairy Sci. 18:131. Warburg, .D. and S. Sakuma. 1923. Pflug. Arch. ges. Physiol., 200:203. Cited from Wilkinson, R. A. 1964. Theories of the mechanisms of oxidized flavor development in dairy.products. Div. of Dairy Res., Comm. Sci. and Ind. Res. Org. Melbourne. Webb, B. H. and A. H. Johnson. 1965. Fundamentals of Dairy Chemistry. The AVI Publishing Co., Inc., Westport, Conn. -78- Wilkinson, R„ A, 1964«, Theories of the mechanisms of'oxidized flavor development in dairy products. Internal Report 4, Div. of Dairy Res., Comm. Sci. and Ind. Res. Org. Melbourne. Wyatt, C. J. and E. A. Day. 1965. Evaluation of antioxidants in deodorized and non-deodorized butteroil stored at 30° C. J. Dairy Sci. 48:682. Zalkin, H., A. L. Tappel and J. P. Jordan. 1960. Studies of the mechan­ ism of vitamin E action. V. Selenite and tocopherol inhibition of lipid peroxidation in chick. Arch. Biochem. Biophy. 91:117. Zittle, C. A. and J. H. Custer. 1963. Purification and some of the properties of -casein and K-casein. J, Dairy Sci. 46:1183. M ONTANA STATE UNIVERSITY LIBRARIES I''- CO 111 111 I ill I Illll 1001 6 75 0 *378 K95 cop.2 Ku, Shun The autoxidative behavior of. "/V37& K35 cop.Z ^oUNOe> I