Second Law of Thermodynamics: Concepts & Applications
advertisement

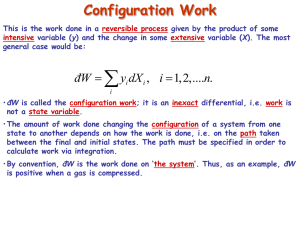
Supplemental Notes for Chapter 4 Second Law: Concepts and Applications Real, Irreversible, Quasi-static, and Reversible Partially quasi-static �� �� �� �� �� Quasi-static Real (Irreversible) Reversible Internally reversible Quasi-static processes - Along a quasi-static path all intermediate states are equilibrium states; thus from postulate I quasi-static paths for closed, simple systems can be described by two independent properties. - From postulate II, if a system progressing along a quasi-static path is “isolated” from its environment, then the values of all properties will remain constant and equal to those just before the isolation. - Quasi-static processes occur at finite rates but not so rapidly that the system is able to adjust on a molecular level. There would not be, in general, gradients of any intensive properties, such as temperature, pressure, density, etc. - Expanding a gas contained in a frictionless piston (mass m, area a)-cylinder is not a quasi-static process: 1. pull stops Pa 2. rapid expansion during which a definite dP/dz gradient exists in the gas phase 3. piston moves rapidly as Pgas is greater than Pgas mg Pa + a ��� 1 Without any friction present, the gas expansion will clearly not be quasi-static. If friction is present so that the expansion process occurs very slowly, dP/dz would be negligible and the properties of the gas would remain constant if the expansion process were stopped – that is, the system would stay in some stable equilibrium state. Thus, with friction present in this manner, the expansion process is quasi-static. A similar situation is encountered in the gas cylinder blowdown. The valve controls the blowdown rate, resulting in a quasi-static process for the gas contained in the cylinder. In fact, the adiabatic tank blowdown process could be modeled as a closed system gas expansion against a massless piston that is frictionally damped to keep Pgas = Poutside. In this case, the process is quasi-static and: d U = − PdV = Pd ( NV ) since N = constant dU = NC v dT = − NPdV for an ideal gas with P = RT/V, by eliminating V, we get dT/T = – R/Cv (dV/V) = –R/Cv ( dT/T – dP/P) (R/Cv+1)dT/T = (R/Cv)dP/P If we had eliminated T, then dP/P =-(1+R/Cv) dV/V and the same equations result by treating the system as open. Upon integration, we obtain the familiar relationships for a reversible, adiabatic expansion (or compression) of an ideal gas, namely, PVκ = constant or equivalently T/Ti = (P/Pi)R/Cp = (P/Pi)(Κ-1)/ Κ where κ ≡ C p / Cv and C p = Cv + R Reversible Processes - Via Postulate II, if any (real or ideal) system in a non-equilibrium state is isolated, it will tend toward a state of equilibrium. - All real or natural processes are not reversible. Hence reversible processes are only idealizations that are very useful in showing limiting behavior. The performance of real processes is frequently compared with ideal performance under reversible conditions. - "In a reversible process, all systems must be in states of equilibrium at all times, that is all subsystems must traverse quasi-static paths." - A system undergoing a reversible process is no more than differentially removed from an equilibrium state – the system passes through a set of equilibrium states. 2 - "A process will be called reversible if a second process could be performed in at least one way so that the system and all elements of its environment can be restored to their respective initial states, except for differential changes of second order." For example, in a reversible expansion or compression δ(δW) ≈ dPdV - If a cyclic process A → B → A is reversible, then when the process is carried out, no changes will occur in any other bodies. For example, if A → B involves the absorption of a quantity of heat Q, then B → A will reject the same quantity Q to the environment. - Any reversible process is also quasi-static, but the reverse is not necessarily true. - Simple systems undergoing reversible processes have no internal gradients of temperature or pressure. - Friction and other dissipative forces are not present in reversible processes. A truly reversible process will always require an infinitesimal driving force to ensure that energy transfer occurs without degradation, hence its rate would be infinitely slow. Therefore, a reversible process always can be shown to require a minimum a mount of work or will yield a maximum amount of work. - Heat engines in reversible processes operate at maximum efficiency Summary of the 2nd Law - The 1st law involves primarily the principle of energy conservation and is not sufficient to describe how a natural process will proceed. - The 2nd law is concerned with describing the direction is which a process can take place. For example, the flow of heat from a hot to a cold body. - The 2nd law describes, in mathematical terms, the physical impossibility of reversing Joule's experiments. It is not possible to convert heat into an equivalent amount of work – some amount of heat must be transferred to a second body or the environment in the process of converting heat into work. - Carnot heat engines operate cyclically and reversibly between two isothermal reservoirs at TH and TC and a work reservoir. The efficiency of the Carnot process for converting heat into work is = – δWc/δQH = (TH−TC)/TH TH>TC so 0 < ηc 1.0 ηc 3 TH δQH δWc δQC TC − Given a reversible process where temperature changes, it is always possible to find a reversible zig-zag path consisting of adiabatic-isothermal-adiabatic steps such that the heat interaction in the isothermal step is equal to the heat interaction of the original process. − Definition of entropy S as an derived state function dS ≡ δQrev / T (an exact differential) ∆ S = ∫ d S and ∫dS = 0 − Clausius inequality for describing heat interactions between two isothermal reservoirs at TA and TB. δQ A / TA + δQB / TB ≥ 0 − For any fully reversible process, the equality applies, for all others the inequality applies − A reversible process adiabatic process occurs at constant entropy. − For any real process; (1) dS > 0 (system + surroundings) (2) ∆ S universe = ∆ S system + ∆ S surroundings > 0 − Entropy is a measure of the degradation of work producing potential. − All natural processes in isolated, closed systems always occur in a direction that increases entropy. 4 Combined First and Second Laws 1. Closed, single phase, simple systems From the 1st Law: dE = dU = δQ + δW For an internally reversible, quasi-static process with only PdV work: δQ = δQrev = Td S and δW = δWrev = − PdV Therefore, dU= TdS−PdV which also provides a way to determine entropy changes: Basis: 1 mole of ideal gas of constant Cp dS=dU/T + P/TdV dS = Cv P dT + dV T T with PV=RT dS = Cv P R dP dT + dT − RT 2 T P T P combining terms: dS = Cv + R dP dT − R T P or 2 T P ∫ d S = ∆S = C p ln T21 − Rln P21 1 5 For ideal gas only 2. Open, single phase, simple systems Insulation For an internally reversible, quasi-static process with one component entering and leaving the system, all intensive properties must remain the same. Hence, �Wrev �nin � Surface (E, V, S, T, P) Tin = Tout = T �Qrev Pin = Pout = P U in = U out = U �nout Sin = Sout = S Adapted from Tester, J. W. and Modell, Michael. Thermodynamics and Its Applications. Upper Saddle River, NJ: Prentice Hall PTR, 1997, p. 87. Vin = Vout = V Image by MIT OCW. From a mole balance, dN = δnin − δnout . Now the 1st Law, which describes an energy balance, can be written with only PdV work as: d E = dU = δQrev − PdV + (U + PV )dN and likewise an entropy balance can be formulated as: d S = δQrev / T + SdN + δ S gen = δQrev / T + SdN since δS gen = 0 for this reversible case. Now by substituting δQrev into the 1st Law expression: d U = Td S − PdV + (U + PV − TS )dN = Td S − PdV + µdN where μ is the chemical potential defined as: μ ≡ G ≡ U + PV − TS = H − TS This result can be generalized for a multicomponent, single phase system that is traversing a quasi-static path as, 6 n d U = Td S − PdV + ∑ µ i dN i i where U is a continuous function of n+2 variables: U = f (S ,V , N i ) i=1,…n and n d U = (∂U / δ S )V , N d S + (∂U / ∂V )S , N dV + ∑(∂U / ∂N i )S ,V , N i j (i ) dN i i T -P Which is often referred to as the Fundamental Equation of Thermodynamics. 3. Availability (maximum and minimum work concepts) As described in Section 14.1, consider a process shown at the right that interacts with a work reservoir and rejects heat to the surroundings. Other constraints are: �nin �nout Primary system Small carnot engine Work reservoir �Qs �Wc Secondary system - reversible/quasi-static operation - steady state δn ≡ δnin = δnout thus δN = 0 for primary system - Carnot heat engine operates cyclicly so δQs is at the system temperature T - all heat δQR is rejected isothermally at T# �Ws �QR Heat reservoir at To Adapted from Tester, J. W. and Modell, Michael. Thermodynamics and Its Applications. Upper Saddle River, NJ: Prentice Hall PTR, 1997, p. 588. Image by MIT OCW. A differential steady state 1st Law balance around the process (primary system) yields: d E = 0 = δQs + δWs + (H in − H out ) δn (1) Where δWs represents the shaft work contribution. A steady state 1st law balance around the Carnot heat engine gives: d E = 0 = −δQs + δQR + δWc Where the – minus sign on δQs reflects its directional change relative to the primary system, i.e., the engine is receiving heat from the system. δQs = δQ R + δWc (2) Where δWc represents the Carnot heat engine work contribution. A steady state entropy balance for the composite secondary system – the process and the heat engine yields: 7 d S = 0 = + δQR / To + (S in − Sout ) δn (3) Combining equations (1), (2), and (3) to eliminate δQs and δQR and solving for the total work interaction (Carnot + shaft work) gives with rearrangement: ∑ δWi = δWtotal = (δWc + δWs ) = [(H out − H in ) − To (Sout − Sin )]δn (4) Since we are dealing with a reversible process, Eq. (4) gives the maximum work per mole that could be produced (or the minimum work required). This is called the availability or exergy: B ≡ H − To S ∆B ≡ H out − H in − To (Sout − Sin ) = ∆H − To ∆S (5) Now Eq. (4) can be rewritten δWmax = (δWc + δWs ) = ∆Bδn (6) or by taking the time derivative to give maximum power: W max = (∆B ) δn / δt = (∆B ) n Clearly, the availability is a state function in the strictest mathematical sense so the maximum (or minimum) work associated with any steady state process is also independent of the path. H:\10.40\2ndlaw.doc (9/12/02) 8 (7)