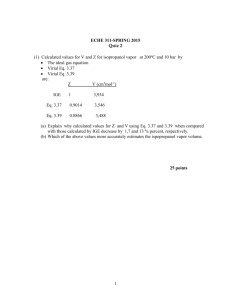
Regulation of IgE antibody responses in mice
advertisement

Regulation of IgE antibody responses in mice by Judith Estelle Klein Manning A thesis submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY In Microbiology Montana State University © Copyright by Judith Estelle Klein Manning (1977) Abstract: The validity of the heterologous (rat) passive cutaneous anaphylaxis (PCA) test to assess mouse IgE responses was confirmed by comparing the 72 hr homologous PCA test with the 24 hr heterologous PCA test, and by the diminution in titer when undiluted serum was heated at 56°C for 90 min. Earlier work, showing that dilution of the serum prior to heating abrogated the effect of heating on the skin sensitizing activity of IgE, was confirmed. Treatment with an anti-helminthic agent improved the homologous, 72 hr PCA reactions in certain mice probably due to a decline In endogenous anti-worm IgE levels as a result of the elimination of pinworms.- Neonatally initiated anti-μ treatment suppressed subsequent IgE responses to Infection with N. brasiliensis showing that IgE forming cells are derived from the same precursors I cell(s) as are IgM, IgG and IgA forming cells. The induction and regulation of IgE responses in Balb/c mice were studied, using the 24 hr heterologous PCA test. Immunization with one intraperitonea 1 (IP) injection of either 1 ù, 10 μg, 100 μg or 1000 μg doses of ovalbumin (OVA), each with 1 mg alum, Induced high PCA and passive hemagglutination (PHA) antibody titers that persisted for at least 56 days. At 100 μg and 1000 μg doses, a lag in the peak IgE response was seen. When B_. pertussis vaccine was used instead of alum, a PHA antibody was Induced but IgE antibody could not be detected. Immunization by one IP injection of DNPn c-OVA + I mg alum Induced a transient anti-DNP IgE response anda persistent anti-OVA IgE response. In contrast, Immunization with DNPgif-OVA induced a persistent anti-DNP IgE response but no ant I-OVA IgE could be detected. Immunization with multiple, IP injections of normal rabbit serum and OVA, without adjuvant, Induced an IgE antibody response. When mice, primed with one IP Injection of 10μg OVA + I mg alum, were given 3, intravenous injections of 100 μg OVA on days 3, 5, and 7 their PCA antibody titers and the number of IgE forming cells In the spleen declined to undetectable levels by day 16, whereas their PHA antibody titers did not change. Secondary IgE responses were also suppressed by this treatment. The route of administration of this treatment was shown to be an important factor In producing this effect. This suppression could be transferred to normal animals by spleen cells from suppressed animals, suggesting that this treatment Induced suppressor cells. REGULATION OF IgE ANTIBODY RESPONSES IN MICE by JUDITH ESTELLE KLEIN MANNING A th e s is sub m itted In p a r t i a l f u l f i l l m e n t o f th e requirem ents f o r th e degree of DOCTOR OF PHILOSOPHY In M ic ro b io lo g y Approved: ys a C h a irp e rs o n , G raduate Committee 7 ft Head^ M ajor Department G raduate Dean MONTANA STATE UNIVERSITY Bozeman, Montana May, 1977 m ACKNOWLEDGMENTS The m ajor p o rtio n o f th e re se a rch f o r t h is th e s is was done o f f campus. T h is was a unique and e d if y in g e x p e rie n c e and I am g r a te ­ f u l t o a l l those whose c o o p e ra tio n made i t p o s s ib le . I w ish t o extend a s p e c ia l thanks t o D r. Norman D. Reed f o r co n se n tin g t o be my m ajor p ro fe s s o r under th ese c o n d itio n s and f o r h is e x te n s iv e support In th e supply o f rese a rch m a t e r ia ls . I am g r a t e f u l , a ls o , f o r th e many e x tr a th in g s th a t he d id so th a t th is arrangem ent could be s u c c e s s fu l. I w is h , a ls o , t o acknowledge th e e x c e lle n t p ro fe s s io n a l example s e t by him . He is a s c h o la r and a good s c i e n t i s t . I wish t o thank D r. David T . Berman, o f th e Departm ent o f V e t­ e r in a r y S c ie n c e , U n iv e r s ity o f W is co n sin , Madison f o r p ro v id in g la b o r a to ry space and equipment th a t a llo w e d me to pursue my th e s is re s e a rc h and f o r h is c o o p e ra tio n , f l e x i b i l i t y and h e lp fu l d is c u s ­ sio n s d u rin g th e tim e I was employed by him . I w ish t o th an k my husband. D r. Dean D. Manning, f o r a ls o p ro ­ v id in g la b o ra to ry space and equipment d u rin g th e f i n a l stages o f my s tu d y , f o r p ro v id in g th e a n tis e ru m f o r our c o lla b o r a t iv e study and f o r h is lo y a l support and encouragement. T h is re se a rch was supported by U .S . P u b lic H e a lth S e rv ic e g ra n ts Al 10384, Al 12854 and CA 17531. Iv TABLE OF CONTENTS Page V I T A . . . ; .............................................................................................................. Ii ACKNOWLEDGMENTS....... ....................................................................................................M i TABLE OF CONTENTS..................................................................................... LIST OF TABLES.................................. iv v ll ABSTRACT................................................................................................. Ix INTRODUCTION................................................................................................................... S tatem en t o f T h e s is ........................................................................................ I 9 MATERIALS AND METHODS........................................................................... 10 A n im a ls ........................................................................................................ 10 A n tig e n s ..................................................................................... A d ju v a n ts .............................................................................................................. 10 II 12 Im m u n izatio n s .................................................................. Serum c o l l e c t i o n ................................................................ 12 A s s ay s ..................................................................................... 13 A n tI-M tr e a tm e n t...................................................................... 15 Worm I n f e c t i o n ...................................................................................... I& RESULTS......................................................................... In d u c tio n o f h cm ocytotropic a n tib o d ie s in m i c e . . . . . ............. 17 The h e te ro lo g o u s ( r a t ) PCA assay f o r mouse Ig E ....... ................. 17 E f f e c t o f an a n t I -h e lm in th ag en t on th e homologous PCA r e a c tio n in ICR m ic e ........................................................................ 18 V Page Suppression o f 8gE a n tib o d y fo rm a tio n by tre a tm e n t w ith a n t i -n a n tis e ru m ........................................................................................ 20 E f f e c t o f m u lt ip le in je c tio n s o f MRS on subsequent IgE a n tib o d y fo rm a tio n t o im m unization w ith ovalbum in and alu m .................................... 24 K in e tic s o f th e PCA and PHA responses o f mice immunized w ith 10 Hg OVA + I mg alum ................................................................. 25 Dose response stud I e s . . . . . ...................................................................... 28 IgE responses t o DNP-conjugates o f o v a lb u m in ........................... 29 Immune response t o v a rio u s doses o f ovalbum in using B. p e rtu s s is as a d ju v a n t........................................................................... 35 PCA a n tib o d y responses t o o th e r a n tig e n s using alum as a d ju v a n t ..................................................................................................... 35 In d u c tio n o f IgE responses by m u lt ip le in je c tio n s o f a n tig e n w ith o u t a d ju v a n t................................ 35 C o n firm a tio n o f p re v io u s f in d in g s ........................... 40 M aintenance o f d e s e n s it iz a t io n ........................................................... 44 E f f e c t o f d e s e n s itiz a t io n on IgE a n tib o d y -s e c re tin g c e l I s .................................. 44 E f f e c t o f g iv in g m u lt ip le , h ig h doses o f a n tig e n w it h ­ o u t alum by th e IP r o u te .................................................. 45 E f f e c t o f g iv in g an o p tim a lly immunogenic dose o f a n t i ­ gen as a d e s e n s itiz a t io n tr e a tm e n t...................................... 46 A comparison o f m u lt ip le and s in g le tre a tm e n ts f o r d e s e n s i t i z a t io n . ...........................................................................................46 E f f e c t o f g iv in g o th e r p ro te in s o r h a p te n -p ro te in con­ ju g a te s In tr a v e n o u s ly ............. ................................. 53 v$ Page E f f e c t o f d e s e n s itiz a t io n tre a tm e n t on secondary IgE responses............ ........................... ................................................. . . 53. S up p ressive e f f e c t o f sp leen c e l l s from s e n s it iz e d d e s e n s itiz e d a n im a ls ........................................................................... 56 DISCUSSION......................... 61 SUMMARY....................... 74 LITERATURE CITED................................................................................ 76 LIST OF TABLES T a b le Page I A comparison between th e homologous and h e te r o lo ­ gous p a s s iv e cutaneous a n a p h y la x is (PCA) te s ts f o r mouse IgE a n tib o d y a g a in s t ovalbum in................................... 21 11 E f f e c t o f h e a t on p a s s iv e cutaneous a n a p h y la x is (PCA) t i t e r s o f mouse a n tis e ru m ....................... ................................22 III IV V Vl Vl I E f f e c t o f an a n ti-h e lm in t h a g e n t on th e homolo­ gous p a s s iv e cutaneous a n a p h y la x is (PCA)r e a c t io n ..................... 23 IgE a n t I -worm responses in m ice t r e a te d from b i r t h w ith a n t i a n t i s e r u m ............................... ............................ . . . . . . 2 6 E f f e c t o f n e o n a ta lI y I n i t i a t e d normal r a b b it serum (NRS) tre a tm e n t on a n t I -o v a lbumin responses in m ic e ........................... 27 Long term m aintenance o f immune responses t o alum adsorbed ovalbum in In B a lb /c m ic e ..................................... 30 IgE responses t o v a rio u s doses o f ovalbum in adsorbed t o alum .................... .31 V lll P as s iv e h e m a g g lu tin a tio n a n tib o d y responses t o v a rio u s doses o f ovalbum in adsorbed to alum .............. . . . . . . . 3 2 IX IgE a n t I -h a p te n and a n t i - c a r r i e r responses o f B a lb /c m ice immunized w ith l i g h t l y d I n l t r o p h e n y la te d ovalbum in (DNPQ g -O V A )............... ; ........................ . . . 3 6 X Xl IgE a n t i h a p te n -c a r r ie r c o n ju g a te , a n ti-h a p te n and a n t i - c a r r i e r responses o f B a lb /c mice Immunized w ith h e a v ily d i n itro p h e n y Ia te d ovalbumin (DNP24 - O V A ) . . . ....................................... A n tI -o v a lbtmiln responses In mice immunized w ith v a rio u s doses o f ovalbum in and B o r t e t e lla p e r t u s s i s . ................................................ ..3 7 38 vlH XH Immune responses o f m ice immunized by neonatal Iy i n i t i a t e d , m u lt ip le , in je c tio n s o f normal r a b b it serum (MRS) w ith o u t a d ju v a n t ............................... ............. 4 1 X lll IgE responses o f m ice immunized by a d u lt i n i t i a t e d m u lt ip le in je c tio n s o f normal r a b b it serum (NRS) w ith o u t a d ju v a n t ........................................................................................42 XIV E f f e c t o f in tra v e n o u s , m u lt ip le in je c tio n s o f s o lu b le a n tig e n on th e a n t I -o v a I bumin responses o f m ice s e n s itiz e d p re v io u s ly w ith an I „pe in ­ j e c t i o n o f a n tig e n + alum .............................. .................... ............. .. .4 7 XV E f f e c t o f in tra v e n o u s , m u lt ip le in je c tio n s o f s o lu b le ovalbum in on IgE r e s p o n s e s . . . . ......................... ............. 48 XVI H eterologous a d o p tiv e cutaneous a n ap h y la x is re a c tio n s in s e n s itiz e d and sensi t I z e d -d e sens i 11 zed m ic e ......................................... ; ................................................49 X V II E ffe c t o f m u lt ip le , in tr a p e r ito n e a I in je c tio n s o f s o lu b le a n tig e n on a n t I -o v a I bumin responses a t day 16 ..........; ............. .................................................................... .. .50 X V III E ffe c t o f g iv in g m u lt ip le in tra ve n o u s in je c tio n , o f low doses o f s o lu b le a n tig e n on d e s e n s it iz a t io n ............ 51 XIX A comparison o f th e e f f e c t o f m u lt ip le in je c tio n s and a s in g le in je c t io n o f ovalbum in on desens I t I z a t i o n . . . . ................................................ .................... ........................... .52 XX E f f e c t o f a d m in is te rin g o th e r p ro te in s o r h a p te n p r o te in c o n ju g ates in tra v e n o u s ly on th e a n t i ­ ovalbum in PCA responses o f m ice p re v io u s ly s e n s itiz e d w ith a I urn-adsorbed o v a lb u m in ............................. . . . 5 4 XXI X X II Secondary responses o f m ice d e s e n s itiz e d w ith m u l t i p l e , h ig h doses o f ovalbum in given in tra v e n o u s ly .............. .....................................................................5 7 S uppressive e f f e c t s o f s p lee n c e l l s from desen­ s i t i z e d anim als on IgE a n t I -o v a I bumin responses when tr a n s fe r r e d t o norma I , syngeneic m i c e . . . . . . ................ 60 ABSTRACT The v a l i d i t y o f th e h e te ro lo g o u s ( r a t ) p a ss iv e cutaneous ana­ p h y la x is (PCA) t e s t t o assess mouse IgE responses was confirm ed by comparing th e 72 hr homologous PCA t e s t w ith th e 24 h r h eterolo gous PCA t e s t , and by th e d im in u tio n In t i t e r when u n d ilu te d serum was heated a t 56 eC fo r. 90 m in. E a r l i e r w o rk, showing t h a t d i l u t i o n o f th e serum p r i o r to h e a tin g a b ro g ated th e e f f e c t o f h e a tin g on th e s k in s e n s it iz in g a c t i v i t y o f Ig E , was c o n firm e d . Treatm en t w ith an a n t i- h e lm in t h ic a g en t improved th e homologous, 72 h r PCA re a c ­ tio n s in c e r t a in m ice p ro b ab ly due t o a d e c lin e In endogenous an­ ti-w o rm IgE le v e ls as a r e s u lt o f th e e lim in a tio n o f pinworms,N e o n a ta lly i n i t i a t e d a n t I tre a tm e n t suppressed subsequent IgE responses t o In fe c t io n w ith N. bras 11 lens is showing th a t IgE fo rm in g c e l l s a r e d e riv e d from th e same p recu rso rs I c e l l ( s ) as a re IgM, IgG and IgA fo rm in g c e l l s . The In d u c tio n and r e g u la tio n o f IgE responses in B a lb /c mice w ere s tu d ie d , using th e 24 h r h e te ro lo g o u s PCA t e s t . Im m unization w ith one in tr a p e r ito n e a I ( IP ) in je c t io n o f e it h e r I fig , IO fig, 100 fig o r 1000 fig doses o f ovalbum in (OVA), each w ith I mg alum . Induced high PCA and p a s s iv e h e m a g g lu tin a tio n (PHA) a n tib o d y t i ­ t e r s th a t p e rs is te d f o r a t le a s t $6 d ays. A t 100 fig and 1000 fig doses, a lag in th e peak IgE response was seen. When Bi, p e rtu s s is v a c c in e was used In s te a d o f alum , a PHA a n tib o d y was Induced but IgE a n tib o d y could not be d e te c te d . Im m unization by one IP in je c ­ t io n o f DNPn c -OVA + I mg alum Induced a t r a n s ie n t a n t i -DNP IgE response a n d a p e r s is te n t anti-O V A IgE response. In c o n tr a s t , Im m unization w ith DNPg^-OVA induced a pars I s t e n t . a n t i -DNP IgE r e ­ sponse but no a n t I -OVA IgE could be d e te c te d . Im m unization w ith m u l t i p l e , IP in je c tio n s o f normal r a b b it serum and OVA, w ith o u t- , a d ju v a n t. Induced an IgE a n tib o d y response. When m ice , prim ed w ith one IP In je c t io n o f 10 fig OVA + I mg alu m , were g iv e n 3 , in tra ve n o u s In je c tio n s o f 100 fig OVA on days 3 , 5 , and 7 t h e i r PCA a n tib o d y t i t e r s and th e number o f IgE form ing c e l l s in th e sp leen d e c lin e d to u n d e te c ta b le le v e ls by day 16 , whereas t h e i r PHA a n tib o d y t i t e r s d id n o t change. Secondary IgE responses w ere a ls o suppressed by t h i s tre a tm e n t. The ro u te o f a d m in is tr a tio n o f t h i s tre a tm e n t was shown t o be an im p o rtan t f a c ­ t o r in producing t h is e f f e c t . T h is suppression could be tr a n s fe rr e d t o normal a n im als by s p le e n c e l l s from suppressed a n im a ls , suggest­ ing th a t t h is tre a tm e n t induced suppressor c e l l s . INTRODUCTION In humans, th e ’ 's k in s e n s it iz in g f a c t o r " p re se n t in th e serum o f hay fe v e r p a tie n ts has been c l e a r l y a d is tin c t i d e n t i f i e d as b elongin g to Immunoglobulin c la s s , d esig n a te d as IgE f o r its a b ilit y t o bind p u r i f i e d ragweed a n tig e n (a n tig e n E) in ra d io im m u n o e le c trc p h o re s is ( I ) . O ther names f o r t h is a n tib o d y in c lu d e : re a g in , homo- c y t o tr o p ic a n tib o d y , and P-K a n tib o d y . IgE d i f f e r s from o th e r known Immunoglobulins in physico ch em ical, a n tig e n ic and b io lo g ic a l p r o p e r tie s . I t c o n ta in s 12% c a rb o h y d ra te , has a s e d im e n ta tio n c o e f f i c i e n t o f 8 . OS and a m o le c u la r w eight o f a p p ro x im a te ly 1 9 0 ,0 0 0 . It Is p re s e n t In serum a t much lower concen­ t r a t io n s than o th e r im m unoglobulins, w ith an average c o n c e n tra tio n o f 0 .3 n g /m l. E le v a te d serum le v e ls a re p re se n t in in d iv id u a ls who a r e a to p ic or in fe c te d w ith worms. E p s ilo n heavy ch ain s have unique a n tig e n ic d e te rm in a n ts and do not possess any o f th e m ajor a n tig e n ic d e te rm in a n ts p re s e n t in th e o th e r fo u r known Immunoglobulin classes (I). The most d i s t i n c t i v e p ro p e rty o f IgE is i t s a b i l i t y to bind to mast c e l l s f o r up t o s e v e ra l weeks a t l e a s t , w ith th e re le a s e o f h is ta m in e and o th e r p h a rm a c o lo g ic a lly a c t iv e substances from these c e l l s upon in t e r a c t io n o f th e c e l l bound IgE w ith a n tig e n ( 1 , 2 ) . T h is p ro p e rty is th e b a s is f o r most o f th e assays f o r t h i s a n tib o d y in anim als and* u n t i l r e c e n t ly , In humans. The e f f e c t s o f h is ta m in e 2 and o th e r pharm acological Iy a c t iv e substances a re th e cause o f th e symptoms exp e rien ce d by a l l e r g i c in d iv id u a ls . T h is c e l l b in d in g a c t i v i t y can be d e stro yed by h e a tin g a t 56°C f o r 2 -4 hours ( I , The dog is th e o n ly o th e r sp ecies 2 ). in which spontaneous a l l e r ­ g ic symptoms s im ila r to those found in hay fe v e r p a tie n ts have been o b served. d e te c te d A s k in s e n s it iz in g f a c t o r s im ila r to human IgE has been in serum from th e s e anim als ( 3 ) . However, heat l a b i l e , h o m o cytotropic a n tib o d ie s which rem ain bound to s k in mast c e l l s f o r long p e rio d s o f tim e ( i . e . , 72 hours) can be d e l ib e r a t e ly induced in many sp ec ie s and a re con sid ered th e anim al c o u n te rp a rt to human IgE ( I , 2, 4 ). These a n tib o d ie s have been d e te c te d in many o th e r mammals in c lu d in g monkeys, dogs, m ice , r a t s , r a b b it s , c a t t l e , sheep p ig s and guinea pigs ( 4 ) , and in a m a rs u p ia l, th e quokka ( 5 ) . The p hysicochem ical p r o p e rtie s o f th e s e hom ocytotropic a n tib o d ie s a re s im ila r to human IgE ( I , 2 , 4 ) and in some cases, c r o s s -r e a c t im­ m u nological Iy w ith anti-hum an IgE ( 6 , 7 ) . . The d e lib e r a t e in d u c tio n o f IgE (c o n tra s te d w ith th e " a to p ic " c o n d itio n ) re q u ire s a s u it a b le com bination o f A n tig e n , dose o f an­ t ig e n , a d ju v a n t, im m unization schedule and species ( 4 ) . It Is gen­ e r a l l y agreed th a t f o r most a n tig e n s some a d ju v a n t is re q u ire d f o r IgE in d u c tio n . A d ju van ts commonly used t o induce IgE responses a re aluminum h y d ro xid e g el (alum ) and v a c c in es o r s o lu b le e x tr a c ts o f B o r d e te ila p e rtu s s is (4 ). and concanavaI i n A (con A) B a c te r ia l I Spopolysaceharides (LPS) ( 8 ) have a ls o been used, as w e ll as F re u n d 9S com plete a d ju v a n t, b u t th e l a t t e r a d ju v a n t f o r IgE (4 )„ ju v a n t -s p e c ie s (4 ) is not co n sid e re d a good . E xperim en tal o b s e rv a tio n s on th e v a rio u s a d ­ in te r a c tio n s suggest t h a t th e mechanisms r e g u la tin g IgE p ro d u c tio n may d i f f e r both f o r sp ec ie s and f o r a d ju v a n t ( 4 ) . One a p p a re n t e x c e p tio n t o t h i s requirem ent f o r a d ju v a n t is th e in d u c tio n o f a n t i- h e lm in t h ic Ig E . Some in v e s tig a to r s b e lie v e th a t l i v e worm in fe c t io n may p ro v id e a d ju v a n t a c tio n because worm ex­ t r a c t s , by th e m s elv es , cannot induce th e IgE response ( 4 ) . Another p o s s ib le e x c e p tio n is th e In d u c tio n o f IgE a n tib o d ie s t o h e te r o lo ­ gous serum by im m unization w ith one in tr a p e r ito n e a I ( I P ) in je c t io n o f h e te ro lo g o u s serum in th e form o f a n ti-ly m p h o c y te serum ( 9 ) . E xp erim en tal e vid en ce has f i r m l y e s ta b lis h e d th a t c o lla b o r a tio n between bone-marrow d e riv e d (B) and th ym us-derived (T ) lymphocytes is re q u ire d f o r th e in d u c tio n o f c e r t a in a n tib o d y responses ( 10) . The im portance o f a t h i r d c e l l ty p e , th e macrophage, has a ls o been reco g n ize d ( 1 1 ) . S e v e ra l lin e s o f e vid en ce dem onstrate th a t s im ila r c e l l u l a r events a r e a ls o necessary f o r IgE p ro d u c tio n as o u tlin e d below . I) Using h a p te n -c a r r ie r system s, i t has been shown th a t T c e l l s a re prim ed w ith c a r r i e r and then ' in t e r a c t w ith B c e l l s t o form h a p te n s p e c if ic IgE in both a d o p tiv e t r a n s f e r experim ents (1 2 -1 5 ) and hi 4 v i t r o experim ents ( 16) . 2) . Rats thymectomlzed w it h in 24 hours o f b i r t h cannot produce IgE a n tib o d ie s 3) . (1 7 ). L e th a l Iy ir r a d ia t e d r a ts r e c o n s titu te d w ith thymus and bone mar­ row c e l l s produce a low le v e l o f IgE whereas 'those r e c e iv in g bone marrow a lo n e do not ( 1 8 ) . 4) C o n g e n ita l Iy athym ic (n u /n u ) m ice cannot produce d e te c ta b le le v ­ e ls o f IgE t o ovalbum in unless r e c o n s titu te d w ith thymocytes (1 9 ) or thymus glands ( 20 ) . Some in v e s tig a to r s have suggested a heterogenous p o p u la tio n o f h e lp e r T c e l l s ; one s p e c if ic f o r IgE and one s p e c if ic f o r O th ers suggest one p o p u la tio n o f h e lp e r I IgG (1 6 ) „ c e l l s f o r both Immunoglobu^ I i n c la s s e s w ith a d i f f e r e n t i a l s u s c e p t i b i li t y to T c e l l re g u la to ry in flu e n c e s o f th e re s p e c tiv e c la s s e s o f a n tib o d y fo rm in g B c e lls (2 1 ). More r e c e n tly a c r i t i c a l d u c tio n o f h e lp e r I c e lls fo r r o l e f o r adherent c e l l s in th e In ­ IgE p ro d u c tio n has been shown ( 2 2 ) . Two d i f f e r e n t p a tte rn s o f IgE a n tib o d y p ro d u c tio n have been re c o g n iz e d . One is t r a n s ie n t , n o n -b o o stab le and u s u a lly th e r e s u lt o f Im m unization w ith a high dose o f a n tig e n . shown In s e v e ra l sp ecies ( 4 ) . T h is p a tte r n has been The o th e r is s u s ta in e d f o r r e l a t i v e l y long p e rio d s o f tim e and b o o s ta b le . T h is p a tte r n is c h a r a c t e r is t ic o f IgE p ro d u c tio n to p o lle n a n tig e n s in humans w ith hay fe v e r ( 2 3 ) , . 5 t o h e lm in th a n tig e n s as a r e s u lt o f p a r a s ite in fe c t io n ( 2 4 ) , and in m ice immunized w ith a low dose o f a n tig e n adsorbed t o alum ( 25 ) . I n t e r e s t in th e management o f c l i n i c a l a ll e r g y has prompted s tu d ie s o f r e g u la to ry mechanisms In IgE a n tib o d y fo rm a tio n . d iv id u a ls w ith hay f e v e r , tite r s In in ­ i t has been shown th a t serum IgE a n tib o d y p e r s is t and a re enhanced a f t e r c o n ta c t w ith a n tig e n durin g hay fe v e r season ( 2 3 ) . H y p o s e n s itiz a tio n tre a tm e n t o f a ll e r g y con­ s is t s o f m u lt ip le in je c tio n s o f m inute doses o f a lle r g e n over long p e rio d s o f tim e . As a r e s u lt o f t h is tre a tm e n t IgG a n tib o d y le v e ls a r e in creased and secondary IgE t i t e r s a r e e v e n tu a lly suppressed. I t was thought th a t t h is tre a tm e n t Induced a b lo c k in g a n tib o d y , p re ­ sumably IgG , th a t competed w ith IgE f o r b in d in g o f a lle r g e n . How­ e v e r , a n a ly s is o f t i t e r s o f b lo c k in g a n tib o d y in c e r t a in tr e a te d pa­ t i e n t s f a i l e d to support t h is h yp o th esis ( 2 3 ) . T h is evidence cou­ p le d w ith th e f a c t t h a t IgG Is not p re s e n t in v e ry g r e a t q u a n titie s in r e s p ir a to r y s e c r e tio n s , th e s i t e where th e y would be most e f f e c ­ tiv e in competing f o r a n tig e n , suggested th a t an In c re a s e in b lo c k ­ ing a n tib o d y may not be re s p o n s ib le f o r th e e f f e c t o f h y p p s e n s itiz a ; t io n on c l i n i c a l symptoms. An a l t e r n a t i v e h ypothesis was suggested, nam ely, th a t a change in th e memory c e l l p o p u la tio n , p a r t i c u l a r l y In T c e l l s , o ccurred w ith th e subsequent suppression o f th e secon­ dary IgE response ( 2 3 ) . 6 The tr a n s ie n t p a tte r n o f IgE a n tib o d y fo rm a tio n In c e r t a in lab o ­ r a to r y a n im als p ro v id ed one means o f s tu d y in g th e suppression o f t h i s c la s s o f a n tib o d y . Using th e r a t as an e x p e rim e n ta l anim al and a unique im m unization schedule to induce IgE , th e te rm in a tin g event in th e tr a n s ie n t p a tte r n o f response was shown to in v o lv e r e g u la tio n a t th e T c e l l le v e l. In t h is m odel, IgE responses to a d in itro p h e n y l c o n ju g a te o f A s c a ris suum e x t r a c t (DNP-Asc) were induced by a high dose o f DNP-Asc ( I mg) in je c te d w ith a B. p e r tu s s is v a c c in e v ia the fo o tp a d s , on day 0 , fo llo w e d by an in tra m u s c u la r In je c t io n o f 0 .5 mg o f DNP-Asc on day 5 ( 2 6 ) . c lin e d by day 2 1 . by T c e l l s IgE t i t e r s peaked by day 14 and then de­ The lin e s o f evidence f o r th e r e g u la tio n o f IgE in t h is system In c lu d e enhancement and m aintenance o f high IgE le v e ls beyond day 21 by a n t i thymocyte serum (ATS) (2 7 ) ; a d u lt thymectomy ( 17)> splenectom y ( 17) and x - i r r a d i a t i o n a d d it io n , ( 28 ) . In i t was shown th a t th e IgE responses prolonged by these means could be suppressed by tr a n s f e r o f sp len o c y te s o r thymocytes from anim als hyper immunized w ith c a r r i e r a n tig e n ( 2 9 ) . O ther c la s se s o f a n tib o d y w ere not s im i l a r ly a f f e c t e d by these tre a tm e n ts . Suppression o f IgE p ro d u c tio n by p a ss iv e a d m in is tr a tio n o f an­ tig e n s p e c if ic IgG a n tib o d y has a ls o been dem onstrated In th e r a t . ( 26 ) and in th e r a b b it ( 30 ) . It is in te r e s tin g to n o te th a t In th e r a b b i t , p a s s iv e a d m in is tr a tio n o f a n t ig e n - s p e c if ic IgM a n tib o d y e n - 7 haneed IgE fo rm a tio n ( 3 1 ) . A somewhat d i f f e r e n t e x p e rim e n ta l model f o r IgE responses e x is ts in th e mouse. When mice a re immunized w ith a low dose o f a n tig e n in co m b in ation w ith alum th e y g iv e a high and p e r s is te n t IgE response (2 5 ). tio n T h is model most c lo s e ly resembles th e p a tte r n o f IgE produc­ in hay fe v e r p a t ie n t s . S e v e ra l examples o f r e g u la tio n o f IgE a n tib o d y fo rm a tio n In t h is model have been dem onstrated. The a b i l i t y o f mice to form IgE a n tib o d y , as w e ll as IgG a n t i ­ body, to low doses o f a n tig e n ( 0.1 jxg -l jug) in alum appears t o be under g e n e tic c o n t r o l, dependent on H-2 ty p e (3 2 ) and comparable to th e ty p e o f Immune response r e g u la tio n observed w ith th e s y n th e tic , branched p o ly p e p tid e s (TG )-AL and (HG)-AL ( 3 3 ) . S t r a in d iffe r e n c e s t o h ig h e r doses o f a n tig e n (100 *ig) were not as a p p are n t ( 3 2 ) , When h a p te n -p ro te in c o n ju g ates were used, t h i s s t r a in d if f e r e n c e was shown t o be r e la t e d t o c a r r i e r p r o t e in , sug g estin g th a t th e g e n e tic c o n tro l o ccu rred a t th e T c e l l le v e l (3 2 ). A nother ty p e o f g e n e tic c o n tr o l, u n re la te d to H-2 ty p e and s p e c if ic f o r 3 4 ). IgE has been observed (3 2 , Thus, th e SJL s t r a in o f mouse produces high t i t e r s o f IgG an­ tib o d y a g a in s t many d i f f e r e n t a n tig e n s bu t low le v e ls o f Ig E , even w ith h ig h doses o f a n tig e n . The IgE t i t e r s o f these m ice can be en­ hanced by using Con A as a d ju v a n t In s te a d o f alum ( 3 5 ) . ly , A d d itio n a l­ I t has been shown t h a t th e low re a g in p ro d u c tio n is due t o th e 8 presence o f n o n -s p e c if ic suppressor I A n o th er means o f r e g u la tin g Is by amount o f a n tig e n . c e l is ( 36 ) . IgE a n tib o d y in th e mouse system Whereas low doses o f alum -adsorbed a n t i ­ gen induce a p e r s is te n t response, high doses o f alum -adsorbed an­ tig e n induce a tr a n s ie n t response ( 2 5 ) . A nother ty p e o f r e g u la tio n has been observed in m ice immunized w ith a low dose o f alum -adsorbed a n tig e n . Maia e t a l , (3 7 ) re p o rte d th e s e le c t iv e d e p ressio n o f IgE a n tib o d y fo rm a tio n t o ovalbum in In D B A /IJ m ice, i f , a f t e r being im­ munized w ith an o p tim a l dose o f ovalbum in ( 0.1 # jg - 1 .0 Mg) and alum, ( I R ) , m ice were g iv e n In tra v e n o u s ( I V ) in je c tio n s o f 100 MQ o f s o lu ­ b le ovalbum in on days 3 » 5 , 7 > t h e i r r e a g in lc response was near n o r­ mal on days 8 , 9 , 10 but had d e c lin e d by day 16. c o n tin u e d t o make r e a g in lc a n tib o d y . C o n tr o l. anim als The IgG response, as measured, by th e 2 hour homologous PCA t e s t , was n o t a f f e c t e d . T h is p ro to c o l is s im ila r to h y p o s e n s itiz a tio n th e ra p y In hay fe v e r p a t ie n t s . Tada (4 ) has suggested th a t t h i s phenomenon may re p re s e n t an example o f to le r a n c e In IgE fo rm in g B c e l l s . Bach and B ra s h le r ( 3 8 ) , re p o rtin g a s im ila r phenomenon produced by m u lt ip le IV in je c tio n s o f a c e ty la te d o v a la lb u m ln , s p e c u la te th a t t h e i r system may re p re s e n t th e f o r ­ m ation o f suppressor T c e l l s . More r e c e n tly i t has been dem onstrated th a t t h i s ty p e o f suppression can be tr a n s fe r r e d t o normal anim als v ia s p le n ic T lymphocytes ( 3 9 ) . In a d d it io n , i t was subsequently shown t h a t th e s e suppressor c e l l s could be g enerated j_n v i t r o only 9 i f a d h ere n t c e lt s were absen t from th e c u ltu r e ( 22 ) . STATEMENT OF THESIS The purpose o f t h is study was t o examine th e r e g u la tio n o f IgE a n tib o d y p ro d u c tio n in m ice and to r e l a t e t h is to c e l l u l a r events^ The study was focused p r im a r ily on th e B a lb /c s t r a in in o rd e r to a v o id th e c o m p lic a tio n s a r is in g from s t r a in d iffe r e n c e s in re g u la ­ t i o n o f IgE s y n th e s is . The e x p e rim e n ta l approach c o n s is te d o f : 1) An exa m in atio n o f th e r e la t io n s h ip o f Ig E -p ro d u c in g c e l l s t o o th e r c la s s e s , o f a n tib o d y -p ro d u c in g c e l l s by te s t in g th e e f f e c t o f n e o n a tal Iy 2) in itia te d ant I on subsequent IgE a n tib o d y responses A comparison o f th e v a rio u s ways in which IgE responses can be induced and r e g u la te d . 3) An a tte m p t t o e lu c id a te th e mechanism(s) re s p o n s ib le fo r th e s e le c t iv e suppression o f IgE responses by th re e in traven o u s In ­ je c t io n s o f high doses o f s o lu b le a n tig e n . MATERIALS AND METHODS A nim als B a lb /c mice were ra is e d in our own la b o ra to ry f a c i l i t i e s . C3H/HeJ m ice were o b ta in e d from Jackson L a b o ra to rie s , Bar H arb o r, M a in e. ICR mice were o b ta in e d from th e Research A nim als Resources C e n te r, U n iv e r s ity o f W is co n sin , M adison. CF^ mice w ere purchased from Carw orth Farms, P o rta g e , M ic h ig a n . O utbred a lb in o r a ts were purchased from ARS Sprague-Dawley or H o ltzm an, C o ., M adison, W isco n sin . A n tig e n s The fo llo w in g a n tig e n s were o b ta in e d from Calbiochem (San D ie g o , C a l i f o r n i a ) : Ovalbum in, (OVA) 5X c r y s ta l 11z e d , and th e fo llo w in g d in ltr o p h e n y la te d (DNP-) p ro te in s : l o t #387039 DNP-o v a I bumin (DNP24 -O V A ), DNP-bovihe serum album in (DNPgg-BSA), DNP-Keyhole lim ­ p e t hemocyanin (DNPgyg-KLH). S u b s c rip ts r e f e r t o th e averag e num­ b er o f groups per m o lecu le o f p r o t e in . In th e case o f KLH, t h is was based on a m o le c u la r w e ig h t o f 2 x IO ^. O ther a n tig e n s and t h e i r commercial sources a r e : normal ra b ­ b i t serum (NRS), C olorado Serum C o .; o v in e a lb u m in , Sigma; ovine gamma g lo b u lin , P en tex . Ragweed e x t r a c t was a g i f t from D r. E. Rau, E ndocrine Labora­ t o r i e s , M adison, W is co n sin . N tp p o stro n q ylu s b ra s ? Iie n s t s a n tig e n was prepared a cc o rd in g to th e method o f O g ilv ie ( 4 0 ) . L i g h t ly d i n Jtro p h en yla te d ovalbum in (DNPooJ-OVA) was prepared by th e method o f E isen (4 1 ) as m o d ifie d by Is h lz a k a and Okudalra (4 2 ). B r i e f l y , 50 mg each o f d I n itro b e n z e n e s u lfo n ic a c id , 2X r e - c r y s t a l l i z e d , and o f sodium c a rb o n a te were d is s o lv e d in 6 ml d is ­ tille d w a te r . To t h i s , 100 mg o f OVA were added and th e m ix tu re s t i r r e d f o r 3 - 1 / 2 h r a t room te m p e ra tu re . The r e s u lt in g c o n ju g ate was d ia ly s e d a g a in s t s a l i n e , a t 4 ° C f o r s e v e ra l days (changed a t le a s t 5 X ). The number o f DNP groups per m olecule o f OVA was d e te r ­ mined s p e c tro p h o to m e trSeal Iy (4 3 ) by m easuring absorbance a t 360 nm f o r DNP and 280 nm f o r OVA. The p r o te in c o n c e n tra tio n was e stim a te d by lo w erin g th e observed OD28 Q re a d in g by 38 . 5% to c o r r e c t f o r th e a b s o rp tio n a t 280 nm by DNP ( 4 3 ) . A d ju v an ts P re p a ra tio n o f A l (0H)^ gel (A lu m ): F if t e e n g o f alum (p u r­ chased from a lo c a l d ru g s to re ) was d is s o lv e d In 180 ml o f d i s t i l l e d w a te r . To t h i s s o lu t io n , 75 ml o f continuous m ix in g . d i s t i l l e d w a te r. IN NaOH was added dropw ise w ith The r e s u lt in g p r e c i p i t a t e was washed 6 - 8X w ith Dry w eig h t d e te rm in a tio n s were done on th e f i n a l s o lu t io n . B o r d e te iIa p e rtu s s is v a c c in e was purchased from El I L i l l y , 12 C o ., In d ia n a p o l I s , In d ia n a (c o n tro l #8AL31A). Im m unizations The s ta n d ard im m unization used t o induce a good IgE response was 10 ng OVA mixed w ith I mg alum g iv e n IR on day 0 . ■ ■ The OVA. was f r e s h ly prepared each tim e , by d is s o lv in g k mg OVA In TOO ml s a lin e . An equal volume o f t h is s o lu tio n was mixed w ith an equal volume o f alum (4 m g /m l), and 0 .5 ml o f t h is m ix tu re was in je c t e d . For those exp erim ents in which a d i f f e r e n t a n tig e n was used o r th e amount o f a n tig e n v a r ie d , a s im i l a r p ro to c o l was fo llo w e d w ith th e a p p ro p ria te , a d ju s tm e n ts . in some e x p e rim e n ts , anim als re c e iv e d , in a d d itio n t o th e s ta n - !; dard Im m u n izatio n , th re e IV In je c tio n s o f a n tig e n w ith o u t a d ju v a n t on days 3 » 5 , 7 . In th e s e e x p e rim e n ts , th e term s e n s itiz e d is used f o r th ose an im a ls r e c e iv in g th e s tan d ard im m unization; th e term sen­ s i t i z e d -d e s e n s itiz e d is used f o r th o se r e c e iv in g th e a d d itio n a l tr e a tm e n t. O th er im m unization p ro to c o ls a r e d e sc rib e d in th e t e x t . Serum co l le c t io n A t th e a p p ro p ria te tim es a f t e r le c te d from th e t a l l Im m unization, blood was c o l­ in to p l a s t i c m ic ro c e n trifu g e tubes to which I drop o f s a lin e had been added. The c lo t t e d blood was rimmed and ' l e f t f o r s e v e ra l hours o r o v e rn ig h t a t 4 ” C. The serum was c o l l e c t - ; I :| ] 13 ed a f t e r c e n t r if u g a t io n and s to re d In 1 /4 or 1/2 dram v ia l s a t -20°C o r -7 0 °C . Serum s to re d In t h is manner d is p la y e d e q u iv a le n t PCA t i t e r s 9 months a f t e r c o l le c t i o n . Assays IgE responses were assayed by th e p a ss iv e cutaneous a n a p h y la x is (PCA) t e s t perfo rm ed , f o r most e x p e rim e n ts , in r a ts (4 4 , 4 5 ) . For each serum, o r serum p o o l, a 0 .1 0 ml a liq u o t o f each o f a s e rie s o f tw o fo ld d i lu t io n s was In je c te d backs o f t e s t r a t s . t i n e l y made. in tra d e rm a l Iy (ID.) in to th e shaved A p p ro xim ately, 40 in je c tio n s per r a t were rou­ The r a ts were c h a lle n g e d 24 hr l a t e r by Intravenous in je c t io n o f 5 mg a n tig e n d is s o lv e d in 0 .5 ml 1% E van's b lu e . When a ss a y in g f o r PCA responses a g a in s t MRS, th e c h a lle n g e a n tig e n con­ s is te d o f 2 ml o f NRS in which 5 mg E van's b lu e dye was d is s o lv e d . S i m i l a r l y , f o r PQA responses a g a in s t N lp p o s tro n q y lu s a n tig e n s , 5 mg o f E van's b lu e dye was d is s o lv e d in 1-2 ml o f . a s a lin e s o lu tio n con­ t a in in g 2 ,0 0 0 worm e q u iv a le n ts . T h i r t y m inutes a f t e r IV c h a lle n g e , th e r a ts were s a c r if ic e d and th e re a c tio n s read on th e underside o f th e s k in . A p o s it iv e re a c tio n was In d ic a te d by a d is c r e t e c i r c l e o f b lu e . For both th e ID and IV In je c t io n s , th e r a ts were sedated by an in tra m u s c u la r in je c t io n o f 0 .0 4 - 0 .0 6 ml I n n o v a r-v e t ( P i t man-Moore C o .). 14 When th e PCA t e s t was perform ed In m ice, 0 .0 5 ml o f a serum d i l u t i o n was in je c t e d , were c h a lle n g e d ID , in to th e shaved backs o f m ice . The mice IV , 72 h r l a t e r , w ith 0 .2 ml 1% E van's b lu e dye, s a lin e , c o n ta in in g 1-2 mg o f a n tig e n . In Only one ID in je c t io n per mouse was made. IgE a n tib o d y -p ro d u c in g c e l l s were measured by th e h eterolo gous a d o p tiv e cutaneous a n a p h y la x is (HACA) t e s t ( 4 6 ) . T h is procedure is s im i l a r to th e PCA assay except th a t 0 .1 ml a liq u o t s o f th e appro­ p r i a t e c e l l suspensions were In je c te d in to th e shaved backs o f r a t s . A n tib o d ie s o th e r than those o f th e IgE c la s s were assayed by p a ss iv e h e m a g g lu tin a tio n (PHA) using e ry th ro c y te s coated w ith OVA. Two d i f f e r e n t methods f o r c o a tin g e ry th ro c y te s were used. In one method ( 4 7 ) , OVA was coupled t o guinea p ig e ry th ro c y te s w ith c a rb o d iim id e . To 3 ml o f PBS in which 30 mg o f OVA was d is s o lv e d was added 0 .0 5 ml o f washed packed guinea p ig e r y th r o c y te s . To th is was added 50 mg I - e t h y l -3 ( 3 -d im e th y la m i n o p ro p y l)-C a r b o d iimide hy­ d r o c h lo rid e (Sigma) d is s o lv e d in 0 .5 ml PBS. T h is m ix tu re was l e f t a t room te m p e ra tu re f o r I hr w ith o c ca s io n al m ix in g . The c e lls w ere then washed 3X and resuspended in m o d ifie d A I s e v ie r 's s o lu tio n (4 8 ) t o a p p ro x im a te ly 0.5% . In th e o th e r method ( 4 9 ) , OVA was coupled t o sheep e r y th r o ­ c y te s w ith chromium c h lo r id e (C r C lg ). (1 0 mg/ml S tock s o lu tio n s o f CrCIg in d i s t i l l e d w a te r) and OVA ( I mg/ml In s a lin e ) were p r e - 15 pa re d „ For th e c o u p lin g p ro c ed u re , th e s to c k CrCl^ s o lu tio n was d ilu t e d 1 /2 0 in s a lin e . To 0 .2 ml o f C rC lg s o lu tio n was added 0 .2 ml o f OVA s o lu tio n and 0 .2 ml washed packed sheep e r y th r o c y te s . T h is m ix tu re was l e f t a t room te m p e ra tu re f o r 5 m in u te s , and then th e c e l l s were washed 4X in s a l i n e . c e l l s were d ilu t e d A f t e r th e la s t wash th e packed 1 /6 0 in m o d ifie d A l s e v i e r gS s o lu tio n and s to re d . For use in assays a f u r t h e r 1/10 d i l u t i o n o f th e c e l l s was made ( f i n a l d i l u t i o n = 1/ 600 ) . PHA t i t e r s were determ ined by m i c r o t it e r using p l a s t i c V-bottom m i c r o t it e r p la t e s , 25 n I d i l u t i n g loops and 25 p i p ip e t t e droppers. M o d ifie d A I s e v i e r gS s o lu tio n was used as a d i l u e n t . A ll t i t e r s a re expressed as th e r e c ip r o c a l o f th e la s t d i l u t i o n t© g iv e a p o s itiv e r e s u lt. A n t l- p tre a tm e n t The a n t i -p a n tis e ru m was p repared by D r. Dean D. Manning, U n i­ v e r s i t y o f W is co n sin , M adison, as d e s c rib e d p re v io u s ly ( 5 0 ) . in 14 hr o f b i r t h , each o f th r e e l i t t e r s was d iv id e d groups. One group In each l i t t e r was in je c te d W ith ­ In to th re e IP w ith 0 .0 5 ml o f a n t i - p serum, th e o th e r two r e c e iv in g an id e n tic a l in je c t io n o f NRS o r p h o s p h a te -b u ffe re d s a lin e (PB S); f u r t h e r in je c tio n s o f 0 .0 5 , 0 . 07 , 0 . 0 7 , 0 . 0 8 , and 0 .0 8 ml were made on days 2 , 4 , 6 , 8 , and 10 , r e s p e c t iv e ly . T h e r e a ft e r a l l m ice re c e iv e d a 0 .1 0 ml in je c t io n o f 16 PBS o r d ilu t e d NRS o r a n t I serum (b o th d ilu t e d 1 :2 .5 in PBS) e v e ry Monday, Wednesday, and F rid a y u n t i l te rm in a tio n o f th e e x p e ri m ent, Worm in fe c t io n A t a p p ro x im a te ly 40 days o f age a l l mice re c e iv e d a subcutane­ ous in je c t io n o f 300 t h ir d - s t a g e ( in f e c t io u s ) adapted s t r a in o f N. bras 11 lens is ( S I ) , la rv a e o f a mouse- 2 wk l a t e r a l l mice were r e in f e c te d w ith 300 such la rv a e and 13 days t h e r e a f t e r were exsan­ g u in a te d r e t r o - o r b i t a l I y . RESULTS In d u c tio n o f hoanocytotropic a n tib o d ie s P rev io u s s tu d ie s in th is in mice la b o ra to ry have e s ta b lis h e d th a t 10 Hg OVA '+ 10 mg alum g iv en IP to B a lb /c mice is v e ry e f f e c t i v e fo r in d u c tio n o f 72 hr PCA a n tib o d y using th e homologous PCA assay ( 2 0 ) . T h is was confirm ed and i t was shown th a t I mg o f alum was j u s t as e f f e c t i v e as 10 mg. 10 days a f t e r in a d d it io n , C3H/HeJ mice gave a PCA t i t e r , a t im m unization, th a t was s im ila r to B a lb /c m ice. The h e te ro lo g o u s ( r a t ) PCA assay f o r mouse IqE A lthoug h IgE is con sid ered a hom ocytotropic a n tib o d y , shown th a t human IgE could bind to monkey mast c e l l s l a r l y , v a rio u s (5 2 ). i t was S im i­ lin e s o f evidence have shown th a t mouse Ig E , but not lgG||, w i l l bind t o r a t mast c e l l s (4 4 , 4 $ , 5 3 , 5 4 ) . ing is s e le c t iv e f o r mouse Ig E , when r a t s , S in ce th is bind in s tea d o f m ice , a re used as th e r e c ip ie n ts f o r th e p a ss iv e t r a n s f e r o f mouse a n tis e ru m in th e PCA assay a la t e n t p e rio d o f 2 -7 2 hours can be employed ( 4 5 ) . A comparison between th e homologous and h etero lo g o u s ( r a t ) PCA assays is shown in T a b le I . S im ila r PCA t i t e r s were observed w ith th e two a s s a y s , a lth o u g h th e t i t e r d ilu tio n In th e homologous assay is one lo w er. To f u r t h e r t e s t th e a b i l i t y mouse Ig E , o f th e r a t PCA assay to, measure I examined th e heat s e n s i t i v i t y o f th e PCA a n tib o d y . It had been re p o rte d p re v io u s ly ( 55 ) th a t d i l u t i n g serum p r io r to heat 18 tre a tm e n t a t 56°C f o r 30 m inutes would p ro te c t th e IgE s k in s e n s i­ t i z i n g a c t i v i t y from h e at d e s tr u c tio n . S ince these w orkers were using th e homologous RCA assay I re te s te d t h is o b s e rv a tio n using th e h e te ro lo g o u s ( r a t ) PCA assay w ith a longer h e a tin g tim e . The h e at tre a tm e n t was c a r r ie d out as fo llo w s : quots were removed from a serum pool from 10 m ice. Two 0 .1 ml a l i ­ One a liq u o t was p la ce d in a 10 x 75 mm t e s t tu b e , corked t i g h t l y and th e tube was th en p laced in a 56°C w a te r bath f o r 90 m in u te s. was d ilu t e d 1:8 in PBS and d iv id e d The o th e r a liq u o t In to 2 p o rtio n s . One o f these was heated as above w h ile th e o th e r was placed in th e c o ld . A fte r th e in c u b a tio n p e rio d , f u r t h e r d ilu t io n s o f th e samples were made and te s te d f o r PCA a c t i v i t y II. in r a t s . The r e s u lts a re shown in T a b le The PCA t i t e r was d im in is h e d from 256 to < 4 f o r pool I and from 2048 to 4 f o r pool 2 by h e a tin g serum b e fo re d i l u t i o n w h ile serum d ilu t e d p r i o r to h e a tin g showed o n ly a s li g h t re d u c tio n in t i t e r . These r e s u lt s c o n firm t h a t th e a n tib o d y measured by th e r a t PCA a s ­ say is h e a t l a b i l e and th a t d i l u t i o n o f th e a n tib o d y p r io r t o heat tre a tm e n t p ro te c ts i t from d e s tr u c tio n . E f f e c t o f an a n ti-h e lm in t h agent on th e homologous PCA re a c tio n in I CR mice A comparison between v a rio u s outb red mice as r e c ip ie n ts fo r th e homologous PCA t e s t produced an in t e r e s t in g fin d in g . I t was noted 19 th a t ICR m ice o b ta in e d from th e Research Anim als Resources C e n te r, U n iv e r s ity o f W is co n sin , M adison, c o n s is te n tly gave v e ry poor or n e g a tiv e PCA re a c tio n s w ith a n tis e ru m th a t produced s tro n g re a c ­ tio n s in CF| mice o b ta in e d c o m m e rc ia lly . J a rre tt e t a l . re p o rte d th a t N ip p o s tro n q y iu s bras 11 le n s is in fe c t io n (5 6 ) have in r a ts had an In h ib it o r y e f f e c t on subsequent homologous PCA ass a ys . I hypothe­ s iz e d th a t a s im ila r e f f e c t due to p I nworm in fe c t io n m ight be r e ­ s p o n s ib le f o r th e e f f e c t on PCA re a c tio n s seen in ICR m ice. A p r e lim in a r y t e s t o f t h is h yp o th esis was made in c o lla b o r a ­ t io n w ith D r. Ralph Anslow , U n iv e r s ity o f W is co n sin . m ice w ere found t o have a high pinworm burden, S ince th e I CR i t was decided to ad m in is t e r an a n ti-h e lm in t h agent t o th e s e mice and t e s t th e e f f e c t o f such tre a tm e n t on t h e i r r e a c t i v i t y in th e PCA a ss a y. A group o f r e t i r e d fe m a le b re ed e r mice was s e p a ra te d from th e main colony and d iv id e d Dodge L a b o r a to r ie s , In to 2 groups. I n c . , F o r t Dodge, A s o lu tio n o f D yrex R (F o rt Iowa) a t a c o n c e n tra tio n o f 2 .5 gm per l i t e r o f w a te r was a d m in is te re d as th e s o le source o f d rin k in g w a te r f o r 14 days t o one group w h ile th e o th e r group was g iv e n p la in w a te r. A p p ro x im a te ly two weeks a f t e r th e tre a tm e n t p e rio d s e v e ra l m ice from each group were s a c r if ic e d and t h e i r t e s tin e s examined f o r p inworms. in ­ The tr e a te d group was a p p a re n tly f r e e o f worms, whereas th e u n tre a te d group had worms. Two months 20 la te r , th e m ice w ere used as r e c ip ie n t s The r e s u lt s fe r e n c e f o r th e hom ologous PCA t e s t . shown in T a b le I I in d ic a t e t h a t th e r e is a d e f i n i t e d i f ­ in th e q u a l i t y and i n t e n s i t y o f th e PCA le s io n between th e tw o g ro u p s . The PCA le s io n s o f th e t r e a t e d g ro u p w ere la r g e r , d a r ­ k e r b lu e and more d is c r e t e th a n th o s e o f th e u n tre a te d g ro u p . th e When in t e s t in e s o f th e s e m ice w ere e xam ine d , p inworms c o u ld n o t be d e te c te d in . e ith e r g ro u p . t y c o u ld n o t be r e la t e d A lth o u g h in t h i s c a s e , th e PCA r e a c t i v i ­ t o pinw orm b urde n a t th e tim e o f th e a ssay t h e r e was a s tr o n g c o r r e l a t i o n betw een a n t i- h e lm in t h tre a tm e n t and PCA r e a c t io n s . th e T h is s t r o n g ly s u g g e s ts t h a t th e p o o r r e a c t i v i t y o f ICR m ice was due t o a worm I n f e c t io n . S u p p re s s io n o f IqE a n tib o d y fo r m a tio n by tre a tm e n t w it h a n t i - u a n tis e ru m I t was o f in t e r e s t to know w hether th e ontog enic development o f IgE form ing c e l l s is r e la te d to IgM b e a rin g p re c u rs o ra l c e l l s , as a r e th e c e l l s form ing o th e r c la s s e s o f im m unoglobulins. way o f s tu d y in g th is r e la t io n s h ip l i n fo rm a tio n by n e o n atal Iy is by suppression o f immunoglobu­ i n i t i a t e d a n t i - # tre a tm e n t. ment has been shown to suppress th e fo rm a tio n o f IgM, and IgA in m ice ( 5 7 ) . One Such t r e a t ­ Ig G ^ IgGg, K ishim o to and Is h iz a k a (5 8 ) showed th a t a n t i - # a n tib o d ie s cannot suppress secondary IgE responses o f r a b b it c e l l s j_n v i t r o . T h e r e fo r e , i t was decided to t e s t th e e f f e c t o f jm 21 TABLE I A comparison between th e homologous and h eterolo gous pa ss iv e cutaneous a n a p h y la x is (PCA) te s ts f o r mouse IgE a n tib o d y a g a in s t ovalbumin Mouse a n t is e r a 3 ______ PCA t i t e r s Mouse Rat I 128 256 Pool 2 128 256 Pool aT i t e r s were done on a serum pool from 3 -5 m ice. Mice were immu­ n iz e d w ith 10 jug ovalbum in + I mg alum IP on day 0 and b le d 10 days la te r . 22 TABLE I I E f f e c t o f h e a t3 on p a s s iv e c u ta n e o u s a n a p h y la x is t it e r s Serum poo1c from 10 mice (PCA)*3 o f mouse a n tis e ru m 1 :8 d i l u t i o n unheated U n d ilu te d s e rum heated 1 :8 d il u t i o n heated I 256 <4 128 Pool 2 2048 4 1024 Pool a 56°C f o r 90 m in u te s. kpCA t e s t perform ed in r a t s . cM ice were immunized w ith 10 fig OVA + I mg alum , IP . 23 TABLE I I I E f f e c t o f an a n t i- h e lm in t h a g e n t on th e hom ologous p a s s iv e c u ta n e o u s a n a p h y la x is (PCA) r e a c tio n PCA a c t i v i t y 9 In t e n s it y C o n tro l^ 2+ + T re ated ^ D iam eter 15 x 15 unmeasurable D e s c rip tio n d is c r e te b a r e ly v i s i b l e 1+ 12 x 10 + 10 x 10 d i f f u s e , s p lo tc h y + 12 x 15 d i f f u s e , S plotchy 2+ 12 x 15 d if f u s e 4+ 20 x 15 d is c r e te 3+ 20 x 25 d is c r e te 3+ 20 x 20 d is c r e te 3+ 20 x 25 d is c r e te d is c r e te aA n tiseru m used f o r p a ss iv e t r a n s f e r was a pool o f serum ob tain ed from mice immunized w ith 10 jug ovalbum in + I mg alum . ^ T re a te d anim als were g iv en Dyrex R in d rin k in g w a te r f o r 14 days. C o n tro l group was l e f t u n tre a te d . A p p ro x im ately 2 months l a t e r th e m ice were te s te d f o r a b i l i t y to show PCA r e a c tio n s . 24 v iv o , a n t I -/it tre a tm e n t on th e a b i l i t y o f mice to g iv e a subsequent IgE a n tib o d y response to 11. b ra s i I ie n s is a tte m p ts to u t i l i z e in fe c t io n (5 9 ). E a rly th e ovalbum in-alum model were f r u s t r a t e d by th e fre q u e n t suppression o f a n t i - o v a I bumin responses by m u lt ip le tio n s o f NRS (see b e lo w ). Dwyer e t a l . in je c ­ W h ile th e s e s tu d ie s were in p ro g re ss , (6 0 ) re p o rte d th a t they f a i l e d to suppress IgE r e ­ sponses to im m unization w ith ovalbum in and alum by neonatal Iy i n i ­ t i a t e d a n t i -M tre a tm e n t. The r e s u lts o f th is study a r e shown in T a b le IV . th a t a n im als r e c e iv in g a n t I-M tre a tm e n t f a i l e d It is c le a r to produce d e te c t­ a b le le v e ls o f PCA a n tib o d y t o worms, whereas those re c e iv in g e it h e r PBS o r NRS made high le v e ls o f s p e c if ic PCA a n tib o d y . m ice were indeed a c t iv e l y in fe c te d w ith worms was determ ined by worm egg counts in th e fe c es s ev e re p a n s p e c ific humoral T h at a l l ( 51 ) . In a d d it io n , th e achievem ent o f immunosuppression as a r e s u lt o f a n t i -/Lt tre a tm e n t was confirm ed by assay o f serum immunoglobulin le v e ls o f IgM, IgG1, IgG2 , and IgA ( 5 9 ) . E f f e c t o f m u lt ip le m a tio n t o in je c t io n s o f NRS on su bse q ue n t IqE a n tib o d y f o r ­ im m u n iz a tio n w it h o v a lb u m in and alum . D u rin g th e s tu d ie s o f th e e f f e c t o f a n t i -/Lt tre a tm e n t on IgE a n tib o d y fo r m a tio n th e p r e lim in a r y o b s e r v a tio n was made, t h a t c o n t r o l a n im a ls r e c e iv in g NRS tr e a tm e n t t h e in IgE re s p o n s e , when t e s t - 25 ed 10 days a f t e r one IP in je c t io n o f OVA and alum , was suppressed. T h is phenomenon was in v e s tig a te d f u r t h e r . fe re n t l i t t e r s in T a b le V . g iven m u lt ip le The r e s u lts from 2 d i f ­ in je c tio n s o f NRS from b i r t h a re shown In a l l anim als re c e iv in g PBS tre a tm e n t, th e PCA and IHA responses were h ig h , whereas in th e NRS tr e a te d anim als most o f th e responses f o r both PCA and PHA a n tib o d y were e it h e r u n d e te c ta b le or s ig n if ic a n tly low ered. However, th e s u p p res s ive e f f e c t was not always p re s e n t to th e same d e g ree . One anim al in th e NRS tr e a te d group showed a PCA t i ­ t e r o f 128 and PHA t i t e r o f 64 which can probably be considered normal and one anim al showed a PCA t i t e r o f 64 and PHA t i t e r o f 16 which is o n ly s l i g h t l y lower than th a t o b ta in e d n o rm a lly but which p ro b a b ly re p re s e n ts a s ig n if ic a n t su p p res s io n . The o th e r responses in t h is group c l e a r l y a re suppressed. Thus, i t was shown th a t NRS tre a tm e n t d id have a su ppressive e f f e c t on th e IgE a n tib o d y response, bu t t h is e f f e c t was v a r ia b le in i t s e xp re s sio n and was not s e le c t iv e f o r IgE; suppression o f PHA responses p a r a lle le d th a t o f IgE . S im ila r r e s u lts have been ob­ served by a n o th e r in v e s tig a to r (G . M. Lang, personal com m unication). K in e tic s o f th e PCA and PHA responses o f mice immunized w ith 10 jug OVA + I mg alum Vaz e t a l . (2 5 ) showed th a t one IP in je c t io n o f a low dose o f 26 TABLE IV IgE a n ti-w o rm responses in mice tr e a te d from b i r t h w ith a n t i -ju a n tise ru m A n ti-w o rm PCA t i t e r s 3 I PBS t r e a t e d *3 NRS tr e a t e d *1 A n t i- u tr e a te d 512 128 <4 512 128 <4 32 128 0 256 256 0 256 512 0 256 32 0 512 0 256 a PCA = p a s s iv e cutaneous a n a p h y la x is assay perform ed in r a t s . ^M ice re c e iv e d e it h e r PBS, NRS or a n t i- M , IP , acc o rd in g to th e f o l ­ low ing schedule: Days 0 , 2 -0 ,0 5 m l; days 4 , 6 - 0 ,0 7 m l; days 8 , 100 .0 8 m l; t h e r e a f t e r , every Monday, Wednesday and F r id a y -0 .1 0 m l. A t a p p ro x im a te ly 40 days a l l mice re c e iv e d 300 t h ir d - s t a g e ( i n f e c ­ t io u s ) la rv a e o f N. b r a s i I i e n s l s . 2 wk l a t e r a l l mice were r e in ­ fe c te d w ith th e same dose o f la rv a e and b le d 13 days l a t e r . 27 TABLE V E ffe c t o f n e o n a ta lIy i n i t i a t e d normal r a b b it serum (NRS) tre a tm e n t on a n t i - o v a I bumin responses in mice Treatm ent® PBS NRS PCA^ PHA^ 512 512 512 256 512 128 256 512 256 512 <4 2 <4 2 <4 4 16 2 64 16 128 64 a M ice re c e iv e d e it h e r NRS o r PBS, IP , acco rd in g to th e fo llo w in g s ch e d u le : Days 0 , 2 -0 .0 5 m l; day 5 - 0 .0 7 m l; day 8 - 0 .0 8 or 0 .1 0 m l; days 13, 18, 2 3 , 2 8 , 3 5 , 3 7 , 4 2 - 0 .1 0 m l. Day 0 = day o f b i r t h . ^Mice were p re b le d on day 3 6 ; immunized on day 37 w ith 10 jug OVA + 10 mg alum , IP ; b le d on day 4 7 . R e s u lts re p re s e n t in d iv id u a l serum t i t e r s , o f 2 d i f f e r e n t l i t t e r s , on day 4 7 . 28 a n tig e n w ith alum was a r e l i a b l e method o f inducing high and p e r­ s is t e n t IgE t i t e r s munized w ith V I, in m ice. T h is was confirm ed f o r B a lb /c mice im­ 10 jug OVA + I mg alum . From th e r e s u lts shown in T a b le i t can be seen th a t PCA a n tib o d y is p re se n t a t peak le v e ls a t day 10 and is m a in ta in e d a t th a t le v e l f o r a t le a s t 5 months. PHA a n tib o d y A is a ls o induced and m a in ta in e d . Dose response s tu d ie s Vaz e t a I . m ice , (2 5 ) have a ls o re p o rte d t h a t , in c e r t a in s tr a in s o f . im m unization w ith a 100 jug dose o f OVA + 10-20 mg o f alum ex­ e r te d a su p p res s ive e f f e c t on IgE responses. In c o n tra s t to the high and p e r s is te n t IgE response d is p la y e d by mice immunized w ith low doses ( 0 .1 - 1 jug) o f alum -adsorbed OVA, mice immunized w ith a hig h dose (100 jug) o f alum -adsorbed OVA showed a somewhat lower ho­ mologous PCA t i t e r th a t d e c lin e d , to v e ry low or u n d e te c ta b le le v ­ e l s , by day 21 . S in ce th e s e in v e s tig a to r s d id not use B a lb /c m ice in t h e i r s tu d y , I te s te d th e e f f e c t o f one in je c t io n o f d i f f e r e n t doses of OVA on th e a n tib o d y responses o f t h is s t r a in to see i f a s im ila r ty p e o f r e g u la tio n could be dem onstrated. In these s tu d ie s , the amount o f alum was kep t c o n s ta n t ( I mg) and th e PCA t i t e r s were as­ sayed by th e h e te ro lo g o u s ( r a t ) PCA t e s t . The r e s u lt s o f th e PCA and PHA t i t e r s a re shown in T a b les V ll 29 and V I I I , 21, r e s p e c tiv e ly . No evidence o f re g u la tio n o f Ig E , a t day is seen a t high doses o f a n tig e n . 1000 4 g, a l l a s li g h t Over th e dosage range o f I - groups show a high t i t e r a t days 21 and 2 8 . There is lag in th e peak IgE response, w ith h ig h e r doses o f a n tig e n , but t h is e f f e c t is gone by day 2 1 . A s im ila r lag has been observed w ith high doses o f c e r t a in o th e r a n tig e n s and may re p re s e n t a t r a n ­ s ie n t n e u t r a liz a t io n o f IgE a n tib o d y by excess a n tig e n . A lag in peak PHA responses was not seen. A second experim ent ( r e s u lt s not shown) confirm ed th e p e r s is ­ te n c e o f th e IgE response, even a t high doses o f a n tig e n ; a t day 5& a f t e r mice were immunized w ith PCA t i t e r s 10 or 1000 #xg OVA + I mg alum , th e o b ta in e d were s i m i l a r , a t both dosage le v e ls , and to the r e s p e c tiv e responses on day 14. day 21 th e re was v e ry l i t t l e It is in te r e s tin g t o note th a t by d iffe r e n c e between th e t i t e r s o b ta in e d , in both th e PCA and PHA a s s a y s , when 10-1000 ng o f a n tig e n was used fo r im m unization. IgE responses to DNP-conjuqates o f ovalbum in S tu d ie s in th e r a t , on IgE a n tib o d y responses to d i n i tro p h e n y - Ia te d A s c a ris e x tr a c ts (DNP-Asc) showed th a t th e le v e l o f anti-D N P IgE was in v e rs e ly r e la te d to th e number o f DNP groups lin k e d to th e c a r r ie r (6 l ) . Thus, im m unization w ith 4 x IO""** moles DNP/mg c a r ­ r i e r produced an ave ra g e an ti-D N P PCA t i t e r o f 13, whereas I x IO"** 30 TABLE Vl Long term m aintenance o f immune responses to alum adsorbed ovalbumin® in B a ib /c mice Type of a n tib o d y Day 10 PCAb 256° 512 512 128 PHAb 128 512 1024 1024 Day 21 Day 56 Day 150 aM ice were immunized w ith one IP in je c t io n o f 10 ng ovalbum in + I mg alum . bPCA = p a s s iv e cutaneous a n a p h y la x is . PHA = p a s s iv e h e m a g g lu tin a tio n . c Each r e s u lt was o b ta in e d from a serum pool from 5 m ice. TABLE V l l IgE responses to v a rio u s doses o f ovalbum in adsorbed to alum Dose o f ovalbum in 8 Day 10 Day 14 Day 21 Day 28 256 b 256 128 256 10 ng 512 512 512 1024 100 Hg 8 64 512 1024 1000 Hg <8 256 512 1024 I ug a M ice were g iven one IP in je c t io n o f 0 .2 5 ml c o n ta in in g th e in d ic a te d amount o f ovalbum in in s a lin e mixed w ith an equal volume o f a s o lu tio n c o n ta in in g I mg alum. ^Each r e s u lt was o b ta in e d from a serum pool from 4 m ice. TABLE V l l l P as s iv e h e m a g g lu tin a tio n a n tib o d y responses t o V ario u s doses o f ovalbum in adsorbed to alum Dose o f ovalbumin® I 49 Day 10 64b Day 14 Day 21 Day 28 256 512 256 10 fig 512 1024 1024 1024 100 49 512 512 1024 2048 I 000 4 g 256 512 512 2048 a M ice were g iv e n one IP in je c t io n o f 0 .2 5 ml c o n ta in in g th e in d ic a te d amount o f ovalbum in in s a lin e mixed w ith an equal volume o f a s o lu tio n c o n ta in in g I mg o f alum. ^Each r e s u lt was o b ta in e d from a serum pool from 4 m ice. 33 moles DNP/mg c a r r i e r gave a t i t e r o f 103. The a n t I - c a r r i e r r e ­ sponses fo llo w e d th e same p a tte r n . C e r ta in s tu d ie s on a n ti-h a p te n and a n t i - c a r r i e r IgE responses in th e mouse have re v e a le d a dichotomy in these responses. Sehon (6 2 ) re p o rte d t h a t when mice w ere immunized w ith Lee and I uq o f h e a v ily d in itr o p h e iiy la te d OVA (DNPgg-OVA) w ith alum th e PCA a n t i ­ body response produced could be e l i c i t e d in th e PCA r e c ip ie n t by c h a lle n g e w ith DNPgg-OVA but not w ith OVA. Is h iz a k a and O kudaira (4 2 ) showed a dosage e f f e c t , th e c a r r i e r , on a n ti-h a p te n r e la te d to IgE responses using a l i g h t l y d i n l t r o - p h e n y la te d c o n ju g a te o f OVA (DNPg ^-OVA) f o r im m unization. In DBA/gJ, m ice which produce high and p e r s is te n t IgE responses to m i­ n u te doses ( 0 . 0 5 - 0 . 2 jxg) o f OVA, th e y showed th a t im m unization w ith low doses o f DNPg ^-OVA produced high and p e r s is te n t a n t i -DNP r e ­ sponses, whereas h ig h e r doses (10 uq) produced much low er t i t e r s , which were t r a n s ie n t . A nti-O VA PCA responses d id not p a r a l l e l th e a n tl-D N P responses; a n t I -OVA a n tib o d y fo rm a tio n was p e r s is te n t alth o u g h th e le v e l o f a n tib o d y was lower a t h ig h e r doses ( 4 2 ) . c a r r i e r , a t a l l doses, r e s u lte d to In a d d it io n , p rim in g w ith in a secondary a n t i - c a r r i e r response im m unization w ith DNPg^-OVA whereas th e an tl-D N P response was enhanced d r a c c e le ra te d when anim als were primed w ith a low dose o f 34 c a r r i e r but suppressed when th e y were primed w ith a high dose ( 4 2 ) . The a n ti-h a p te n and a n t i - c a r r i e r to IgE responses o f B a ib /c mice im m unization w ith e it h e r DNPq ^-O V A o r DNP2Zfl-OVA w ere compared using th e h e te ro lo g o u s ( r a t ) PCA a s s a y . When mice were immunized w ith DNPq ij-OVA and I mg alum (T a b le IX) t h e i r a n ti-D N P responses were tr a n s ie n t and in v e rs e ly r e la te d t o dose. The a n t I -OVA r e ­ sponses were high and p e r s is t e n t , s im ila r to th a t o b ta in e d w ith un­ c o n ju g ated OVA, except t h a t I *zg DNPq ^-OVA produced a 4 - f o l d h ig h ­ e r t i t e r than 100 p g . When mice were immunized w ith DNPg^-OVA and I mg alum (T a b le X) th e o b s e rv a tio n o f Lee and Sehon (6 2 ) was confirm ed and extended. A nim als immunized w ith 1-100 #g o f a n tig e n produced both a n t i- D N P ^ - OVA and a n ti-D N P responses th a t were high and p e r s is t e n t . However, a n im a ls Immunized w ith 1000 pg o f a n tig e n show no d e te c ta b le a n t i ­ body. Anti-O VA a n tib o d y could not be d e te c te d in any o f th e ex­ p e rim e n ta l groups. When a 1 /8 d i l u t i o n o f an a n t I -OVA serum th a t had d is p la y e d a t i t e r o f 256 upon c h a lle n g e w ith ovalbum in, was p a s s iv e ly tr a n s ­ fe r r e d t o a r a t , th e subsequent c h a lle n g e w ith 5 mg DNP2Zt-OVA was a b le t o e l i c i t a PCA response f o r th a t a n tis e ru m . th a t a t T h is le a s t some o f th e B c e l l a n tig e n ic d e te rm in a n ts were p re s e n t on th e DNP2Zii-OVA m o le c u le . in d ic a te s f o r OVA 35 Immune response to v a rio u s doses o f ovalbum in using f3. p e rtu s s is as a d ju v a n t B a lb /c mice were g iv e n one IP In je c t io n o f 0 .2 5 ml o f a s o lu ­ t io n c o n ta in in g 10, 100, o r 1000 tig OVA + 0 .2 5 ml o f B. p e rtu s s is v a c c in e e q u iv a le n t to 10*0 organism s. T a b le X I . The r e s u lts a re shown in M ice so immunized produced a PHA a n tib o d y , but a p p a re n t­ ly no PCA a n tib o d y . PCA a n tib o d y responses to o th e r a n tig e n s using alum as a d ju v a n t O th er a lu m -p r e c ip ita te d substances which were a b le to Induce PCA a n tib o d ie s , a t days 14 and 2 8 , by im m unization w ith one IP in ­ j e c t i o n w ere: I) ragweed a n tig e n a t doses o f I 4 9 , 10 /tig, and 100 p g , and 2) NRS and normal sheep serum when 0 .2 5 ml o f e it h e r u n d i­ lu te d o r d ilu t e d 1:5 in s a lin e was used. When u n d ilu te d serum was used, a la g In th e peak response was seen s im ila r to th a t observed w ith h ig h doses o f OVA. Im m unization w ith 10 jizg, 100 jug, or 1000 fig doses o f o v in e album in o r OGG p r e c ip it a t e d w ith alum d id not p ro ­ duce d e te c ta b le le v e ls o f PCA a n tib o d y . In d u c tio n o f IqE responses by m u lt ip le In je c tio n s o f a n tig e n w ith ­ o u t a d ju v a n t It is g e n e r a lly accepted th a t In o rd e r t o Induce IgE responses in la b o ra to ry a n im als an " a d ju v a n t" is re q u ire d . th is is th e in d u c tio n o f s p e c if ic An e x c e p tio n to IgE a n tib o d y as a r e s u l t . o f worm TABLE IX IgE a n ti-h a p te n and a n t i - c a r r i e r responses o f B a lb /c mice immunized w ith l i g h t l y d in itr o p h e n y la te d ovalbum in (DNPq ^-OVA) PCA t i t e r s *1 A n ti -DNP Dose o f a n t i qena LA U N I Aig Day 14 A n ti--ovalbum in Day 36 Day 14 <8 2048 512 Day 36 10 /tig 64 <8 512 256 100 Aig <8 <8 64 128 aMice were given one IP in je c tio n of 0.25 ml of the indicated dose of DNPq r=0VA in s a lin e mixed w ith an equal volume of a solution containing I mg alum. kpCA (p a s s iv e cutaneous a n a p h y la x is ) te s ts were perform ed in r a ts which were c h a l­ lenged IV w ith 5 mg o f e it h e r DNP-BSA o r ovalbum in. c Each r e s u lt was o b ta in e d from a serum pool from 3 m ice. TABLE X IgE a n ti h ap te n -c a rrier conjugate, an ti-h ap ten and a n t i- c a r r ie r responses of Balb/c mice immunized w ith h e a v ily di n i trophenylated ovalbumin (DNPg^-OVA) PCA t I t e r s b A n ti DNPgjt-OVA Dose o f a n tig e n 3 A n ti -DNP Day 14 Day 56 128° 128 256 10 Mg 256 128 100 jug 256 1000 wg <2 I M9 Day 14 Day 56 A n ti ovalbum in Day I 4 Day 56 256 <8 <8 256 256 <8 <8 256 >8 128 <8 <8 <4 <4 <4 <8 <8 aH ic e were g iv e n one IR in je c t io n o f 0 .2 5 ml o f th e in d ic a te d dose o f DNPq 5 -OVA in s a lin e mixed w ith an equal volume o f a s o lu tio n c o n ta in in g I mg alum . bpCA (p a s s iv e cutaneous a n a p h y la x is ) t e s ts w ere perform ed in r a ts which were c h a l­ lenged IV w ith 5 mg o f e i t h e r DNPgjt -OVA, DNP-BSA o r ovalbum in. c Each r e s u lt was o b ta in e d from a serum pool from 4 m ice. TABLE Xl A n ti-o v a lb u m in responses in m ice immunized w ith v a rio u s doses o f ovalbum in arid B o rd e te i Ia p e rtu s s is Day 10 Dose o f ovalbum in 3 PCA PHA CTl a O <4b 100 Hg 1000 fig Day 21 Day 14 PCA PHA PCA 16 <4 NDc <4 <4 32 <4 ND <4 128 <4 ND Day 28 PCA PHA 16 <4 ND <4 16 <4 ND <4 128 <4 ND PHA aM ice were g iv e n one IP I n je c t io n o f 0 .2 5 ml ovalbum in in s a lin e mixed w ith an equal volume EL p e r tu s s is v a c c in e e q u iv a le n t to a p p ro x im a te ly 10 ' " organism s. ^ R e s u lts were o b ta in e d from a serum pool from 3 m ice. cNot done. 39 in f e c t io n ( 4 0 ) . One r e s u l t o f l i v e worm in fe c t io n may be th e r e ­ peated re le a s e o f low doses o f worm a n tig e n s d u rin g th e v a rio u s stag es o f th e in f e c t io n . to p ic p e o p le . S im ila r a n t Ig e n lz a tio n may occur in a - I th e r e fo r e c o n sid ered th e p o s s i b i l i t y th a t re p e a t­ ed Im m unization w ith a n tig e n a lo n e m ight overcome th e requirem ent f o r a d ju v a n t in th e in d u c tio n o f IgE responses. Two d i f f e r e n t im m unization p ro to c o ls were used. The f i r s t was s ta r te d on th e day o f b i r t h and g iv en a p p ro x im a te ly e ve ry 2-3 days as o u tlin e d in T a b le X I I . From th e r e s u l t s , it is a p p aren t th a t a h e a t l a b i l e , PCA a n tib o d y response could be e l i c i t e d by neonatal Iy in it i a t e d , m u ltip le , IP , in je c tio n s o f NRS. o f p r e c i p i t a t i o n a n tib o d y were a ls o form ed. ished th e PCA t i t e r , R e la t iv e ly high le v e ls Heat tre a tm e n t d im in ­ but not th e p r e c i p i t i n t i t e r . However, th e re seemed to be a p o rtio n o f th e PCA a n tib o d y th a t is r e s is t a n t to h e a tin g . A s im ila r response was o b ta in e d when mice re c e iv e d neonatal Iy i n i t i a t e d m u lt ip le in je c tio n s o f low doses o f ovalbum in (0 .0 2 -1 0 Mg) a t a p p ro x im a te ly w eekly I n t e r v a l s . A pool o f serum from 7 mice gave an a n t i - o v a I bumin t i t e r o f 64 a t day 4 8 , 7 days a f t e r th e la s t i n je c t io n . In th e second p ro to c o l th e im m unizations were i n i t i a t e d a d u lt a n im a ls . in A t o t a l o f s ix In je c tio n s o f 0 .2 5 ml was a d m in is ­ 40 IP a t v a rio u s in te r v a ls . The mice were assayed a t day 18 , 4 days a f t e r th e la s t in j e c t i o n . The r e s u lt s , shown in T a b le X l l l te re d were s im ila r t o those o b ta in e d w ith n e o n atal Iy i n i t i a t e d t io n s . in je c ­ A pool o f serum from group I was te s te d f o r h e at s e n s i t i v i ­ t y ; th e u n tre a te d pool gave a t i t e r o f 128 , whereas th e heated pool gave a t i t e r o f < 16 , a d d it io n a l I t was o r i g i n a l l y thought th a t by g iv in g an Im m unization o f OVA + alu m , t o group I I , t o s tim u la te a n ti-o v a lb u m in responses, th e a n t I -NRS response m ight have been po­ te n tia te d . However, w it h in th e tim e course o f t h is experim ent th is d id not occur and in f a c t , th e re appears to be a s l i g h t suppression. T hese r e s u lt s c l e a r l y in d ic a t e t h a t , in m ic e , m u l t i p l e in je c ­ t io n s o f a n t ig e n , w it h o u t an a d ju v a n t, can e l i c i t PCA resp on se s t h a t m ost p r o b a b ly a r e o f th e IgE c la s s . The fo llo w in g experim ents w ere designed t o exam ine, d e ta il, th e phenomenon re p o rte d by Maia e t a l . (3 7 ) in more in which mul­ t i p l e high doses o f a n tig e n , w ith o u t alu m , given IV , re s u lte d in th e s e le c t iv e suppression o f IgE responses. C o n fir m a tio n o f p re v io u s f in d in g s B a lb /c mice w ere s e n s itiz e d on day 0 w ith one IP in je c t io n o f 10 ug OVA + I mg alum fo llo w e d by a d e s e n s itiz in g tre a tm e n t, on days 3» 5 , 7 which c o n s is te d o f th e in tra ve n o u s In je c t io n o f e it h e r s a lin e (c o n tro l a n im a ls ), 100 fig OVA w ith o u t alum or 1000 pg OVA 41 TABLE Xl I Immune responses o f mice immunized by n e o n a tal Iy I n i t i a t e d , m u lt ip le , In je c tio n s o f normal r a b b it serum (MRS) w ith o u t adjuvant® A n tI-N R S re sp on se s PCAb U n tre a te d H ea te d c Unheated H eatedc 32 32 ND I 64 2 128 i Mouse no. P r e c ip itin 64 3 64 16 64 64 4 64 16 32 32 5 32 4 64 64 6 16 8 64 64 CL 8 aM ice re c e iv e d NRS, IP , acc o rd in g t o th e fo llo w in g sch ed u le: Days 0 , 2 - 0 .0 5 m l; day 5 - 0 .0 7 m l; day 8 - 0 .0 8 or 0 .1 0 m l; days 13 , 18, 2 3 , 2 8 , 3 5 , 3 7 , 4 2 - 0 .1 0 ml (day 0 => day o r b i r t h ) . They were bled on day 3 6 . kpCA = p a s s iv e cutaneous a n a p h y la x is perform ed in r a t s . cU n d ilu te d serum samples were heated a t 56°C f o r 90 m in u te s. dNot done. 42 TABLE X l l l IgE responses o f mice immunized by a d u lt I n i t i a t e d m u ltip le in je c tio n s o f normal r a b b it serum (NRS) w ith o u t a d ju v a n t AntI-WRS PCA responses 3 Group I *5 128 128 16 64 Group I l k* c 32 8 32 64 a PCA = p a s s iv e cutaneous a n a p h y la x is perform ed in r a t s . a r e from sera c o lle c te d on day 18 . R esu lts ^ H ic e in both groups re c e iv e d 0 .2 5 ml NRS. IP , on days 0 , 2 , 4 , 7 , 11 and 14 (day 0 =» day o f f i r s t I n j e c t i o n ) . cMice in group 11 re c e iv e d , OVA + I mg alum on day 9 . in a d d it io n , one IP In je c t io n o f 10 pg 43 w ith o u t alum each day. These 2 dosages were te s te d because I was using 10 -1 OOX g r e a te r s e n s it iz in g dose f o r B a lb /c m ice than had been used in th e o r ig in a l s tu d y . As can be seen from th e re s u lts In T a b le X IV , tre a tm e n t w ith both 100 ng and 1000 ng OVA re s u lte d in th e suppression o f th e PCA response by day 2 1 , w h ile th e group r e c e iv in g s a lin e had high t i t e r s . W ith th e 100 ng dose, th e IgE r e ­ sponse is a t near normal le v e ls on day 10 , whereas w ith th e 1000 jug dose th e response is much lo w e r, p ro b a b ly in d ic a tin g th a t th e sup­ p re s s io n was expressed a l i t t l e sooner w ith th e h ig h e r dose. The PHA response d id not appear t o be s i m i l a r l y a ffe c te d by th e t r e a t ­ m ent. Subsequent experim ents w ith a s im ila r design w ere c a r r ie d o u t. 100-200 ng OVA f o r th e d e s e n s itiz in g tre a tm e n t was r o u t in e ly used. From th e r e s u lts o f th e s e experim ents i t can be concluded th a t d e s e n s it iz a t io n is a r e p e a ta b le , v a l i d e xp e rim e n ta l f in d i n g . hundred o r 200 /ug Is e q u a lly e f f e c t i v e One in inducing d e s e n s itiz a t io n . By making comparisons between d i f f e r e n t e xp e rim e n ts , i t appeared t h a t te r m in a tio n o f th e IgE response occurred by day 16 and r e ­ mained a t le a s t through day 2 4 . A ls o , th e e xp ressio n o f desensI - tiz a t .io n appeared to be p ro g re s s iv e . A t day 9 th e PCA t i t e r s were n o rm a l, a t day 10 some m ice began t o show a lower PCA t i t e r and by day 11 most anim als showed s ig n if ic a n t supp ressio n . M aintenance o f d e s e n s itiz a t io n When serum from th e anim als discussed above was te s te d a t 77 days a f t e r s e n s i t i z a t i o n , th e PCA responses o f th e groups re c e iv in g e ith e r 100 fig o r 1000 jzg OVA were s t i l l <8 w h ile th e c o n tro l group was s t i l l making PCA a n tib o d y . was long l i v e d . T h is suggested th a t th e phenomenon In a n o th e r e x p e rim e n t, sponses were te s te d , in which In d iv id u a l r e ­ i t was seen t h a t th e d e s e n s itiz e d s t a t e was n o t u n ifo rm ly m a in ta in e d in a n im als t r e a te d w ith 100 ixg OVA. c a l r e s u lt s a re shown In T a b le XV. m a ls , which re c e iv e d m u lt ip le , T y p i­ Bt can be seen t h a t in some a n i ­ IV in je c tio n s o f 100 fig OVA, PCA a n tib o d y was d e te c ta b le on day 52 even though t h e i r response had p r e v io u s ly dropped to u n d e te c ta b le le v e ls by day 16. T h is has been observed in o th e r e x p e rim e n ts . E f f e c t o f desens I t I z a t ion on IqE a n tib o d y -s e c re tin g c e l l s In o rd e r to d e te rm in e w hether th e suppression o f IgE a n tib o d y responses induced by tre a tm e n t w ith m u lt ip le high doses o f a n tig e n was due t o suppression o f a n tib o d y s e c re tio n a n d /o r fo rm a tio n or to n e u t r a li z a t i o n o f a n tib o d y by excess a n tig e n , th e WCA t e s t (34) was em ployed. For t h i s t e s t , an im a ls were s a c r if ic e d a t v a rio u s tim es a f t e r s e n s it iz a t io n and s in g le c e l l suspensions o f t h e i r s p le e n s , thymuses and bone marrows p repared in PBS. The c e l l p re ­ p a ra tio n s were washed 2X w ith PBS and resuspended t o an a p p ro p ria te 45 - c o n c e n tra tio n . made. • ; ■ . . V ia b le c e l l counts by Trypan b lu e e x c lu s io n were T w o -fo ld d i lu t io n s o f t h i s p re p a ra tio n were made and 0 .1 ml a liq u o t s were in je c te d in to th e s k in o f r a ts which were c h alle n g ed 24 h r l a t e r w ith E van's b lu e dye and a n tig e n . The r e s u lts using c e l l s from s e n s itiz e d anim als a r e shown In T a b le X V I. IgE s e c re tin g c e l l s w ere p re se n t in th e spleens and bone marrows, bu t not th e thymuses o f s e n s itiz e d m ice a t days 17 and 3 2 . T h is c onfirm ed p re v io u s work ( 3 4 ) . The r e s u l t s , using c e l l s from d e s e n s itiz e d a n im a ls , a re shown in T a b le X V i. i t can be seen th a t a t th e c e l l c o n c e n tra tio n s , te s te d , no IgE s e c re tin g c e l l s were d e te c te d in e it h e r s p le e n o r bone marrow. C o n sid erin g t h a t th e number o f c e l l s which gave a n e g a tiv e r e a c tio n was 100f o l d g r e a te r f o r sp leen c e l l s and 5-10 f o ld g r e a te r f o r bone marrow c e ils than th e le a s t number o f c e l l s , from th e s e n s itiz e d group, t h a t gave a p o s it iv e r e a c tio n , th e s e r e s u lts s tro n g ly suggested t h a t th e suppression o f IgE re s p o n s e s ,d u e t o in tra ve n o u s in je c ­ t i o n o f a n tig e n , was th e r e s u lt o f a la c k o f IgE a n t I b o d y -s e c re ting c e l l s and not to n e u t r a liz a t io n o f a n tib o d y by excess a n tig e n . ■E f f e c t o f g iv in g m u l t i p l e , high doses o f a n tig e n ,w ith o u t alum by th e IP ro u te From th e r e s u lts shown In T a b le X V I I , I t can be seen th a t when 3 In je c tio n s o f high doses o f a n tig e n a re g iv e n IP in s te a d o f IV , 46 th e IgE a n tib o d y responses a re o n ly s l i g h t l y d im in is h e d when com­ pared w ith th e responses o f a n im als re c e iv in g th e h ig h .d o ses IV . T h is is t r u e when e it h e r je c tio n s . These r e s u lts 100 /ug o r 1000 /ug was used f o r th e IP in ­ in d ic a te th a t th e ro u te o f a d m in is tr a tio n o f th e d e s e n s itiz in g tre a tm e n t was v e ry Im p o rta n t. Thus, th e e x ­ t e n t o f Suppression by IP in je c t io n was not n e a rly as g r e a t as th a t Induced by a low dose o f a n tig e n g iv en in tra v e n o u s ly (s e e b e lo w ). E f f e c t o f g iv in g an o p tim a lly immunogenic dose o f a n tig e n as a desens I t i z a t ion tre a tm e n t T a b le X V M I shows th e responses o f anim als g iv en 10 <Ltg OVA IV , compared t o 100 pg OVA, IV . I t can be seen th a t 10 ng was a b le to induce a s ig n if ic a n t suppression o f PCA t i t e r s . T h is supports th e h y p o th e s is t h a t an Im p o rta n t re q u ire m e n t f o r d e s e n s itiz a t io n is th e ro u te o f in j e c t i o n . A comparison o f m u lt ip le and s in g le tre a tm e n ts f o r desens 51 5za.t I on T a b le XIX shows th e e f f e c t o f g iv in g one IV in je c t io n o f 300 Ug o f OVA on e it h e r day 3 , day 5 , o r day 7 . one s in g le in je c t io n I t can be seen th a t induced a s ig n if ic a n t suppression in PCA t i ­ te r s b u t not as g re a t as th a t o b ta in e d w ith m u lt ip le in je c t io n s . T h e re d i d n 't seem to be much d if f e r e n c e among s in g le in je c tio n s g iv e n on d i f f e r e n t days. 47 TABLE XIV E ffe c t o f in tra v e n o u s , m u lt ip le in je c tio n s o f s o lu b le a n tig e n on th e a n t I -ova I bumin responses o f mice s e n s itiz e d p re v io u s ly w ith an l . p . in je c t io n o f a n tig e n + alum Day 10 T reatm en t Day 0 - Days 3 , 5 , 7 PCA Sens 111z e d -s a 11nea 512 d Sens 11 1zed-desens 11 1zed^ 100 fig Sens 111ze d -d e s e n s i 1 1zedc 1000 uq Day 21 PHA PCA PHA 64 256 512 128 512 <4 256 32 512 <4 512 a M ice were s e n s itiz e d on Day 0 by an in tr a p e r ito n e a I in je c t io n o f 0 .2 5 ml ovalbum in in s a lin e mixed w ith an equal volume o f a s o lu ­ t i o n c o n ta in in g I mg alum . On days 3 , 5 , 7 they were given an in ­ traven ous in je c t io n o f s a lin e . ^M ice were tr e a te d as above except th e y were given 100 /ng s o lu b le ovalbum in in s te a d o f s a lin e on days 3 , 5 , 7 . cM lce were tr e a te d as above, except th e y were g iv en 1000 ug s o lu ­ b le ovalbum in on days 3 , 5 , 7 . ^ R e s u lts were o b ta in e d from a serum pool from 3 m ice. 48 TABLE XV E f f e c t o f In tra v e n o u s , m u lt ip le in je c tio n s o f s o lu b le ovalbum in on IgE responses T reatm en t S e n s itiz e d 3 Day 10 Day 16 Dav 52 256 1024 1024 1024 64 1024 256 512 512 512 1024 256 512 256 512 512 256 256 S e n s itiz e d d e s e n s itiz e d 6 128 128 64 128 256 64 128 32 32 16 8 512 512 512 128 128 256 256 128 512 256 256 256 <8 <8 <8 <8 <8 <8 <8 <8 <8 <8 <8 64 64 32 32 32 32 16 <8 <8 <8 <8 aM ice were s e n s itiz e d on day 0 by an l . p . in je c t io n o f 0 .2 5 ml ovalbum in In s a lin e mixed w ith an equal volume o f a s o lu tio n con­ t a in in g I mg alum . ljM lce were tr e a te d as above, except th e y were g iv en th re e l . v . je c t io n s o f 100 Mg egg album in w ith o u t alum on days 3 , 5» 7 . In ­ 49 TABLE XVI H eterologous a d o p tiv e cutaneous a n a p h y la x is re a c tio n s in s e n s itiz e d and s e n s ltlz e d -d e s e n s I t l z e d mice No. o f c e l l s re q u ire d f o r a p o s it iv e re a c tio n Day 17 Day 32 S e n s itiz e d 3 Spleen 7 .5 X 105 4 .6 x IO5 Bone marrow 6 .0 X IO6 2 .5 x IO6 >5 X IO7 >3 x IO7 Spleen > 5 .2 X IO7 > 6 .2 x IO7 Bone marrow > 3 .2 X IO7 > 3 .5 x 10? Thymus > 4 .5 X IO7 >2 x IO7 Thymus Sens i 11 zed-desens 111 zed*1 a M ice were immunized w ith one IP In je c t io n o f 10 fig OVA + I mg alum on day 0 . ^Mlce were immunized w ith one IP in je c t io n o f 10 ^g OVA + I mg alum on day 0 and su bsequently g iv en th r e e 100 fig doses o f OVA w ith o u t alum on days 3 , 5 , 7 . 50 TABLE XVM E ffe c t o f m u lt ip le , ln t r a p e r lt o n e a l in je c t io n s o f s o lu b le a n tig e n on a n t i - o v a lb u m in re sp on se s a t day 16 T reatment® PCA PHA S e n s it iz a t io n + s a lin e 256 1024 128 2048 256 2048 <4 2048 <4 1024 4 4096 128 1024 64 4096 64 4096 256 4096 64 4096 32 2048 S e n s it iz a t io n + 100 ng IV S e n s it iz a t io n + 100 ng IP S e n s it iz a t io n + 1000 ng IP a On day 0 , a l l mice w ere g iv en one IP in je c t io n o f 10 jug OVA + I mg alu m . On days 3 , 5 , 7 th e y were t r e a te d as In d ic a te d . i, 51 TABLE XVI I I E f f e c t o f g iv in g m u lt ip le in tra ve n o u s in je c t io n o f low doses o f s o lu b le a n tig e n on d e s e n s itiz a t io n A nt?-ova I bumIn responses^ Treatm ent® S e n s it iz a t io n + 100 ug IV S e n s it iz a t io n + 10 fig IV PHAd Exp. I 512 256 256 ND ND ND Exp. 2 256 128 256 1024 2048 2048 CX a S e n s it iz a t io n + s a lin e PCAc <4 <4 <4 ND ND ND Exp. 2 <4 <4 <4 2048 1024 4og6 Exp. I 64 32 16 ND ND ND Exp. 2 8 16 32 4096 4096 4096 aA l l m ice were s e n s itiz e d on Day 0 w ith one IP in je c t io n o f 10 ixg OVA + I mg alum . On days 3* 5 , 7 th e y were given in traven o u s in ­ je c t io n s o f e it h e r s a l i n e , 100 /zg OVA o r 10 /ug OVA. ^ R e s u lts a r e from In d iv id u a l mice b le d on day 16 (E xp. (E xp . 2 ) . c PCA = p a s s iv e cutaneous a n a p h y la x is . clPHA = p a s s iv e h e m a g g lu tin a tio n . I) o r 19 52 TABLE XIX A comparison o f th e e f f e c t o f m u lt ip le In je c tio n s and a s in g le i n je c t io n o f ovalbum in on d e s e n s itiz a t io n T reatm ent3 D av(s) o f tre a tm e n t PCA responses on day I 6 S a lin e 3, 5, 7 512, 128, 256 100 jug OVA 3, 5, 7 < 4 , < 4 , <4 300 jug OVA 3 6 4 , 16, 16 300 jug OVA 5 3 2 , 6 4 , ND 300 ug OVA 7 128, 3 2 , 8 aA l l tre a tm e n ts were g iv e n IV . On day 0 , a l l mice were g iv en one IR In je c t io n o f 10 jug OVA * I mg alu m , . 53 E f f e c t o f g SvSng o th e r p ro te in s o r h a p te n -p ro te in co n ju g ates in - t ravenous Iy In o rd e r to d e te rm in e w hether th e suppression observed in sen­ s i t i z e d mice th a t were g iv en th r e e subsequent IV in je c tio n s o f 100 Ug OVA m ight p o s s ib ly be due t o a n o n -s p e c ific e f f e c t , perhaps s im ila r t o t h a t observed w ith m u lt ip le substances were in je c te d in je c tio n s o f MRS, o th e r IV In a s im i l a r p r o to c o l. The r e s u lt s , shown in T a b le XX, showed th a t n e ith e r 100 ng sheep album in nor 100 fig DNP-KLH had any s u p p res s ive e f f e c t on th e PCA responses of m ice p re v io u s ly s e n s itiz e d w ith 10 jug OVA + I mg alum . were e q u iv o c a l when 100 jug DNPg^-OVA was g iv e n . The r e s u lts One mouse out of th r e e showed a s ig n if ic a n t suppression o f PCA a c t i v i t y , whereas th e mean PCA v a lu e f o r th e o th e r two was o n ly one tube low er than th a t f o r th e a n im als g iv e n no tre a tm e n t. However, even DNPg^-OVA was not a b le t o suppress th e PCA responses t o th e e x te n t t h a t 100 jug OVA c o u ld . E f f e c t o f desens i t I z a t ion tre a tm e n t on secondary IgE responses The e f f e c t o f d e s e n s itiz a t io n on a subsequent IgE secondary response was te s te d by g iv in g a second IP In je c t io n o f 10 jug OVA + I mg alum t o s e n s itiz e d and s e n s itiz e d -d e s e n s itiz e d m ice . The sec­ ond Im m unization was a d m in is te re d a t two d i f f e r e n t In t e r v a ls a f t e r th e p rim a ry I n j e c t i o n . The r e s u lts a re shown In T a b le X X I. When a TABLE XX E f f e c t o f a d m in is te rin g o th e r p ro te in s o r h a p te n -p ro te in conjugates in tra v e n o u s ly on th e a n t i - o v a lbumln PCA responses o f mice p re v io u s ly s e n s itiz e d w ith alum -adsorbed ovalbum in T re atm en t3 on days 3 , 5 , 7 PCA responses*’ on day 16-21 Experim ent I 256 S a lin e <8 100 ng OVA 256 100 ng sheep album in Experim ent 2 S a lin e 100 Hg OVA 100 Hg DNP2lt-OVA 100 Hg DNP-KLH 128, 5 1 2 , 256 < 4 , < 4 , <4 128, 128, 32 5 1 2 , 5 1 2 , 512 3On day 0 , a l l m ice re c e iv e d 10 Hg OVA + I mg alu m , IP . On days 3 , 5 , 7 th e y re c e iv e d , in tra v e n o u s ly , th e In d ic a te d substances. ^ R e s u lts f o r e xperim ent I a r e from a serum pool from 3 m ice c o l le c t ed on day 16. R e s u lts f o r exp erim en t 2 a re from in d iv id u a l serum samples c o lle c te d on day 2 1 . secondary im m unization was g iv en to s e n s itiz e d a n im als on day 29 (Group I ) th e re was an 8 - f o l d in c re a s e in RCA t i t e r a t f i r s t , which i decreases one d i l u t i o n l a t e r on. m in is te re d on day 50 (Group I I I ) te r. When th e second c h a lle n g e was ad­ th e re was a 4 - f o l d In c re a s e in t i ­ I do not c o n s id e r th a t th e s e in creases a re d i f f e r e n t from each o th e r , however, th e y do re p re s e n t s ig n if ic a n t in c re a se s when com­ pared t o th e group th a t d id not r e c e iv e a second im m unisation (Group II). On th e o th e r hand, th e p a tte r n o f secondary responses was somewhat d i f f e r e n t in sens 111ze d -d e s e n s i t Ized mic e . In Group IV , w hich re c e iv e d a second im m unization on day 2 9 , th e PCA Increased by o n ly one d i l u t i o n , whereas in Group V I , which re c e iv e d a second im m unization on day 5 0 , th e PCA t i t e r th e t i t e r was s t i l l s ig n if ic a n tly sponse in th e s e n s itiz e d group. increased 3 2 - f d l d . However, lower than th e re s p e c tiv e r e ­ In Group V , which d id not re c e iv e any secondary im m u n izatio n , th e PCA t i t e r Increased s l i g h t l y be­ tween days 29 and 5 0 . Thus, i t appears t h a t . In d e s e n s itiz e d a n im a ls , th e e x te n t o f in c re a s e in secondary PCA t i t e r s depends on when th e second immuni­ z a tio n was g iv en in r e la t i o n t o th e I n i t i a l s e n s it iz a t io n and desens itlz a tlo n tre a tm e n t. The secondary response was h ig h e r , when th e t im e .i n t e r v a l was In creased between d e s e n s itiz a t io n tre a tm e n t and secondary c h a lle n g e . T h is was, a p p a r e n tly , not tr u e f o r anim als 56 which had been s e n s itiz e d o n ly . The PHA re sp o n se s d id n o t seem t o be s i m i l a r l y ( d a ta n o t sh o w n ). A l l g ro u p s r e c e iv in g a s e c o n d a ry c h a lle n g e showed a s i g n i f i c a n t c re a s e was s i m i l a r in flu e n c e d in c re a s e in t i t e r and th e e x te n t o f t h a t in ­ in a l l c a s e s . S u p p re ss ive e f f e c t o f sp leen c e l l s from s e n s itiz e d -d e s e n s itiz e d an im als Experim ents were undertaken t o see i f th e t r a n s f e r o f spleen c e l l s from s e n s itiz e d -d e s e n s it iz e d a n im als to normal anim als would suppress th e subsequent IgE response o f th e r e c i p i e n t . Two s ep a r­ a t e experim ents were s e t up. In e xperim ent I , 2 groups o f donors were s e n s itiz e d on day 0 w ith 10 ^g OVA + I mg alum . 7 one group re c e iv e d an in traven o u s On days 3» 5 , In je c t io n o f 100 %g OVA a lo n e . A t day 18, th e a n im als were b le d and t h e i r sera assayed f o r PCA a c tiv ity . A t day 19, 4 anim als from th e s e n s itlz e d -d e s e n s itIzed group which had u n d e te c ta b le le v e ls o f PCA a n tib o d y (< 4 ) and 4 a n i ­ mals from th e s e n s itiz e d group (PCA > 64) were s a c r if ic e d and s in g le cell suspensions o f t h e i r spleens w ere prepared in PBS c o n ta in in g 1% f e t a l c a l f serum. A f t e r I wash In t h is medium, th e c e l l s from each group were pooled and a v ia b le count was made. 2 .5 x 10® c e l l s in je c te d A p p ro x im ately in a volume o f 0 . 3 5 - 0 .5 0 m l, from each group, were IV in to normal r e c ip ie n ts which were immunized th e f o llo w - 57 TABLE XXI Secondary responses o f mice d e s e n s itiz e d w ith m u lt ip le , high doses o f ovalbum in g iv e n In tra v e n o u s ly Group I T reatm en t Day o f second im m unization^ S e n s it iz a t io n 8 29 PCA a n t i - o v a I bumin responses Day 29 Day 36 Day 50 Day 57 256d 2048 1024 1024 11 SI NONE 256 512 512 512 111 Il 50 512 512 512 2048 29 64 128 128 128 NONE <4 8 16 16 50 <4 16 16 512 IV S e n s it1 z a tio n -b desens i t I z a t ton " V Vl Il aA l l m ice re c e iv e d 10 Hg OVA * I mg alu m . bA I l m ice re c e iv e d 10 jitg OVA + I mg alum , OVA, w ith o u t alum , IV,, on days 3 , 5 , 7 . IP , on day 0 . IP , on day 0 and 100 Jjtg c H lc e re c e iv e d e it h e r s a lin e or 100 txg OVA + I mg alu m , IP , on th e day in d ic a te d . R e s u lts a r e from a serum pool from 3 m ice. 58 Ing day w ith 10 ng OVA + I mg alum . The a n t I -OVA IgE a n tib o d y r e ­ sponses o f th e r e c ip ie n ts a r e shown In T a b le X X II. I t can be seen t h a t th e PCA responses o f a n im als r e c e iv in g d e s e n s itiz e d c e l l s a re s ig n if ic a n tly c e lls . lower than those o f a n im als r e c e iv in g s e n s itiz e d When th e means o f th e Iogg v a lu e s f o r th ese two groups w ere a n a ly ze d by S tu d e n t's t t e s t , th e d iffe r e n c e s between th e two groups w ere s t a t i s t i c a l l y s ig n if ic a n t (p < 0 . 05 ) . In th e second e x p e rim e n t, 3 groups o f donors were used. groups w ere primed on day 0 w ith 5 , 7 one group re c e iv e d 10 pg OVA + I mg alu m . On days 3 , IV in je c tio n s o f 100 ^g OVA and a n o th e r group re c e iv e d IV In je c tio n s o f 1000 iug OVA. c e iv e d no tre a tm e n t. A ll The t h i r d group r e ­ A t day 19, th e spleens and thymuses o f a l l 3 groups were prepared in a manner s im i l a r t o th a t d e s c rib e d above. F o r each group o f donors, a s p leen c e l l suspension c o n ta in in g 2 .5 x 10® o r 2 .5 x 10? c e l l s in 0 .5 ml was prepared and in je c t e d , In to normal r e c ip ie n t s . A d d i t i o n a l l y , a n o th e r group o f r e c ip ie n ts IV , O re c e iv e d an IV i n je c t io n o f 2 x 10° thymocytes from th e same group o f donors. The a n t I -OVA PCA responses o f r e c ip ie n ts re c e iv in g s p lee n c e l l s a r e shown in T a b le X X iI (E xperim ent 2 ) . The PCA r e ­ sponses o f a n im als re c e iv in g 2 .5 x 10® sp leen c e l l s from anim als t r e a te d w ith 1000 ug OVA were 8 - f o l d low er than those o f anim als r e c e iv in g th e same number o f s e n s itiz e d c e l l s . When 1 0 -fo ld less 59 c e l l s from th e same donors were t r a n s f e r r e d , th e PCA responses were 4 -fo ld low er than those o f s e n s itiz e d a n im a ls , however th e re were o n ly 2 a n im als l e f t in t h is e x p e rim e n ta l group. O The PCA responses o f th e 2 m ice re c e iv in g 2 .5 x 10 from donors d e s e n s itiz e d w ith sp leen c e l l s 100 jug doses o f OVA w ere s im ila r to th o se o f e xp e rim en t I and s i g n i f i c a n t l y m ice r e c e iv in g s e n s itiz e d c e l l s . lower than th e responses o f When 1 0 -fo ld les s c e l l s from t h is group w ere t r a n s f e r r e d , th e s u p p res s ive e f f e c t was not seen. o When 2 x 10 thymocytes from each o f th e th re e groups were tr a n s fe r r e d th e PCA responses o f serum pools from 3 r e c ip ie n ts fo r each gro u p , were id e n t ic a l (PCA t i t e r ° 256 in a l l 3 c a s e s ). These r e s u lts showed th a t th e suppression o f IgE by 3 , IV in ­ je c t io n s o f a n tig e n was t r a n s f e r r a b le to normal m ice by spleen c e l l s , but not by thym ocytes. - : 60 . ■ TABLE XXI I S u p p ressive e f f e c t s o f sp leen c e l l s from d e s e n s itiz e d anim als on IgE a n t i - o v a lbumin responses when tr a n s fe r r e d t o norm al, syngeneic mice T re atm en t o f donors o f s p lee n c e l Is a No. c e l l s t r a n s fe r r e d PCA responses o f r e c ip ie n ts ^ '_______ Day 14-15__________ S e n s itiz e d 2 .5 x 10® 256, 512, 256 Sensi t iz e d -d e s e n s it iz e d -100 *tg 2 .5 x 10® Experim ent I 32, 128 , 64 Experim ent 2 S e n s itiz e d S e n s itiz e d -d e s e n s it iz e d -100 ug S e n s it iz e d -d e s e n s it iz e d - 1000 ng 2 .5 x 10® 5 1 2 , 5 1 2 , 512 2 .5 x IO 7 5 1 2 , 5 1 2 , 512 2 .5 x 10® 128, 6 4 , ND 2 .5 x IO 7 2 56 , 2 5 6 , 512 2 .5 x 10® 6 4 , 6 4 , 64 2 .5 x IO 7 128, 128, 128 aA l I m ice w ere g iv en 10 tig OVA + I mg alu m , IP , on day O0 In th e s e n s itiz e d -d e s e n s it iz e d groups, m ice w ere g iv en 3 , IV in je c tio n s o f th e in d ic a te d doses o f OVA on days 3 , 5 , 7 . ®0ne day a f t e r c e l l t r a n s f e r , a l l + I mg alu m , IP . r e c ip ie n ts re c e iv e d 10 ng OVA DISCUSSION The s t u d ie s on th e h e te ro lo g o u s ( r a t ) PCA t e s t c o n firm e d p re ­ v io u s f in d i n g s and h e lp e d e s t a b lis h th e v a l i d i t y o f t h i s a ssay f o r mouse Ig E e In a d d i t i o n , th e I n h i b i t o r y e f f e c t o f d i l u t i o n on th e h e a t s e n s i t i v i t y o f th e m ast c e l l b in d in g a b i l i t y was c o n firm e d and e x te n d e d . o f PCA a n tib o d y In th e s tu d y by C la u se n e t a l . th e PCA a n tib o d y re s p o n s e was Ind u ced by im m u n iz a tio n w it h (5 5 ) 125 ug OVA p lu s a s a lin e e x t r a c t o f B. p e r t u s s is and a ssa ye d by th e hom olo­ gous PCAe My s t u d ie s e x te n d e d t h i s o b s e r v a tio n t o PCA a n tib o d y i n ­ duced by im m u n iz a tio n w it h a lu m -a d s o rb e d a n tig e n and a ssa ye d by th e h e te ro lo g o u s PCA a s s a y . a 30 m in h e a tin g t im e . A ls o , s in c e C la u sen e t a l . (5 5 ) em ployed I t m ig h t be a rg u e d t h a t by d i l u t i n g o u t th e a n t ib o d y , a lo n g e r h e a tin g tim e w o u ld be r e q u ir e d t o a c h ie v e th e same e f f e c t . My s t u d ie s showed t h a t even when th e h e a tin g tim e was e x te n d e d t o 90 m in th e e f f e c t was s t i l l se e n . A re c e n t paper (6 3 ) has re p o rte d th a t th e re a r e 2 a n tib o d ie s in mouse a n tis e ru m , a c t iv e in th e h etero lo g o u s ( r a t ) PCA assay when a 2 h r l a t e n t p e rio d was used, one h e a t -s ta b le and one h e a t - l a b i l e . (The h e a t -s ta b le a n tib o d y d id not re a c t w ith e it h e r a n ti-Ig G ^ or a n t i Ig G g .) T h is c o n c lu s io n was based on I ) a la c k o f c o r r e la t io n o f 48 h r h o m ocytotropic a n tib o d y a c t i v i t y w ith 2 h r h e te r o c y to tr o p ic ( r a t ) a c t i v i t y , 2 ) th e presence o f a h e at r e s is t a n t a n tib o d y t h a t is more a c t iv e In th e h e te ro lo g o u s assay and 3 ) th e presence o f h e te r e c y to - 62 t r o p ic ( r a t ) a n tib o d ie s a n tib o d y a c t iv e in mouse a n tis e ru m known not t o c o n ta in PCA in th e 48 hr homologous a ss a y. I t should be noted th a t in th e s e s tu d ie s , th e h e at tre a tm e n t was a p p lie d t o a 1:5 d i l u ­ t i o n o f serum which could account f o r th e presence o f th e 1'h e a t-r e ­ s is t a n t " a n tib o d y in some o f th e a n tis e r a te s te d . The d iffe r e n c e s between th e 48 h r h cm ocytotropic and 2 h r h e te r o c y to tr o p ic a n tib o d y w ere n o t more than 2 -4 f o l d . r e s is t a n t PCA a n tib o d y The a p p are n t g r e a te r amount o f heat in th e 2 h r h etero lo g o u s assay when compared w ith th e 4 8 hr homologous assay can be accounted f o r by th e d i f f e r ­ ence in tim e . I t has been shown t h a t a lth o u g h th e h a l f - l i f e o f IgE a n tib o d y in th e s k in was a p p ro x im a te ly 7 -1 4 days ( I , 6 4 ) , th e g r e a t­ e s t r a t e o f decrease occurred w ith th e f i r s t 3 - 4 days ( I ) . The p re ­ sence o f a PCA a n tib o d y In serum from SJL m ice, th a t was a c t iv e in . th e 2 h r h e te ro lo g o u s bu t not th e 4 8 h r homologous t e s t and which appears t o be t o t a l l y h e a t r e s i s t a n t , is n o te w o rth y . However, th ese in v e s tig a to r s f a i l e d t o compare 48 h r homologous assay r e s u lts w ith 4 8 hr h e te ro lo g o u s assay r e s u l t s . l a t e n t p e rio d was employed. In a l l o f my s tu d ie s , a 24 hr I b e lie v e th a t o th e r s tu d ie s (4 4 , 4 5 ) , as w e ll as my own, have e s ta b lis h e d th e v a l i d i t y o f th e h e te r o lo ­ gous ( r a t ) PCA assay t o measure mouse IgE a n tib o d y . The a m e lio r a tio n o f th e PCA le s io n caused by IgE a n tib o d y , in ICR m ice , due t o tre a tm e n t w ith an a n t i- h e lm in t h ic a g e n t has Im p o r- 63 tanfc c o n s id e ra tio n s f o r th e use o f th e PCA assay. It is v e ry l i k e ­ ly th a t th e poor PCA r e a c t i v i t y observed b e fo re tre a tm e n t was due t o a c o m p e titiv e i n h ib it io n o r b lo c k in g o f th e b in d in g o f th e pas­ s iv e l y tr a n s fe r r e d worm I n f e c t io n . IgE by endogenous IgE produced as a r e s u lt o f Is h iz a k a e t a l . (6 5 ) have shown, v e ry c o n v in c in g ly , t h a t when p e r ito n e a l mast c e l l s were incubated w ith '^ ! - la b e le d r a t E myeloma p r o t e in , th e number o f IgE m olecules combined w ith mast c e l l s Is o la te d from N. b ra s H I e n s l s In fe c te d r a t was about 10% o f t h a t bound to mast c e l l s from normal r a t s . The d e te c tio n o f p Inworms Sn th e e xp e rim e n ta l a n im als b e fo re tre a tm e n t suggests s tro n g ly th a t t h i s p a r a s ite was re s p o n s ib le fo r th e e f f e c t seen. A lthoug h i t has not been re p o rte d , i t seems re a ­ so n ab le t o assume t h a t , based on o th e r p a r a s ite systems ( 66 ) , fo rm a tio n occurs as a r e s u lt o f p Inworm In f e c t io n . It IgE Is f e l t th a t th e o b s e rv a tio n s re p o rte d h e re may re p re s e n t an extrem e case f o r th e fo llo w in g reasons: I) th e anim al c olony from which th e e x p e r i­ m ental a n im als were o b ta in e d was n e ith e r m onitored nor tr e a te d f o r p inworms; 2 ) because r e t i r e d fe m a le breed mice were used, th e worm burden and hence, t h e i r IgE le v e ls may have been e x c e p tio n a lly high due to th e In h ib it o r y e f f e c t s o f pregnancy and l a c t a t io n on worm e x ­ p u ls io n ( 67 ) . The o b s e rv a tio n th a t measurements o f PCA a c t i v i t y may be i n - 64 f Iuenced by p inworm in fe c t io n is e x tre m e ly Im p o rtan t c o n s id e rin g th e u b iq u ity o f pinworm In fe c tio n s In anim al c o lo n ie s . However, some commercial anim al s u p p lie rs do m o n ito r t h e i r c o lo n ie s f o r p I nworms and a p p ly a p p r o p r ia te means t o c o n tro l th e in fe c t io n s . The suppression o f IgE a n tib o d y fo rm a tio n by n e o n a ta lIy in i­ t i a t e d tre a tm e n t w ith a n t i - u a n tis e ru m p ro v id es c o n v in c in g evidence th a t Ig E -fo rm in g c e l l s , l i k e those producing IgM, Ig A , a r is e from IgM b e a rin g p re c u rs o r® I c e l l s . c o u rs e , t h a t th e a n t I Ig G j, It IgGg and Is p o s s ib le , o f ser um used in th e s e s tu d ie s c o n ta in ed un­ d e te c te d tra c e s o f a n t i - £ a c t i v i t y which m ight be re s p o n s ib le f o r th e observed s u p p res s io n . T h a t s it u a t io n seems u n l i k e l y , however, in v ie w o f th e degree o f p u r it y o f IgM used to induce fo rm a tio n o f th e a n tis e ru m and th e subsequent e x te n s iv e a b s o rp tio n o f t h i s a n t i ­ serum w ith o th e r im m unoglobulins. e f f e c t is I t seems more l i k e l y th a t th e in f a c t due t o a n t i a n t i b o d i e s . p re s s io n o f IgE re sp o n s iv en e s s, lik e I t may be th a t sup­ IgA s u p p res s io n , re q u ire s a r e l a t i v e l y high le v e l o f s u p p re s s iv e tre a tm e n t. T h is could account f o r th e la c k o f IgE suppression observed by Dwyer e t a l . ( 6 0 ) . The supp ressio n o f anti-O V A responses by n e o n a ta lIy NRS tre a tm e n t proved t o be v a r ia b le known what in flu e n c e s t h i s . It in itia te d in I t s exp re s sio n and i t is not Is c le a r however, t h a t th e e f f e c t Is not r e s t r i c t e d t o th e PCA response b u t extends t o th e PHA response 65 in a p a r a l l e l m anner. g e n ic c o m p e titio n th e c e i l s T h is e f f e c t may be due t o some ty p e o f a n t i ­ (6 8 ) o r t o a d i r e c t e f f e c t , p erha p s c y t o t o x ic , on r e q u ir e d f o r th e g e n e r a tio n o f an immune re s p o n s e . t h e r s tu d y F u r­ is needed. - The r e s u lts o f th e s tu d ie s on im m unization o f B a lb /c mice w ith one IP in je c t io n o f low doses ( I and 10 jug) o f OVA and I mg alum c o n firm ed th a t t h is procedure was a h ig h ly r e l i a b l e means o f induc­ ing h ig h and p e r s is te n t le v e ls o f IgE a n tib o d y . The r e s u lt s , when h ig h e r doses o f OVA (100 and 1000 ug) w ith I mg alum were used, showed th a t t h is procedure a ls o Induced a high and p e r s is te n t IgE response. These r e s u lts d i f f e r e d from th a t o f Vaz e t a t . ( 2 5 ) , who showed t h a t h ig h doses o f OVA Induced a low and tr a n s ie n t IgE r e ­ sponse. The d iffe r e n c e s observed, may be due to d iffe r e n c e s s t r a in s o f mouse used a n d /o r t o d iffe r e n c e s used. Vaz e t a l . In th e in th e amount o f alum (2 5 ) used SW- 5 5 , SWR/J and C57BL/6J mice and 10- 20 mg alum w ith h ig h e r doses o f OVA whereas I used B a lb /c mice and I mg alum . From in fo rm a tio n in th e Handbook o f E xperim en tal munology ( 69 ) i t appears th a t a p p ro x im a te ly 50 49 o f p r o te in maximum amount th a t can be p r e c ip it a t e d by I mg alum . when I Immunized w ith im­ is th e T h e re fo re , 100 o r 1000 yg o f OVA + I mg alu m , th e a n i­ mals were p ro b a b ly re c e iv in g a p p ro x im a te ly 50 49 o f alum p r e c ip i­ ta te d a n tig e n + 50 o r 950 pg o f n o n -p re c Ip it a t e d a n tig e n . 66 Presumably th e n , th e alum p r e c ip it a t e d a n tig e n remained as a depot w it h in th e a n im a l, whereas th e r e s t o f the a n tig e n , th e nonp r e c ip it a t e d p o r t io n , a c te d as any o th e r s o lu b le a n tig e n and be­ came a v a i l a b l e to th e c i r c u l a t i o n , c r e a tin g a high c ir c u l a t i n g con­ c e n t r a t io n o f a n tig e n , which was then m e ta b o lize d and e x c re te d nor­ m a lly . T h e r e fo r e , th e a c tu a l dose o f a n tig e n th a t p e rs is te d w ith in th e depot was p ro b a b ly about 50 u g f no m a tte r how much more was g iv e n i n i t i a l l y w ith I mg alum . T h is may not have been enough an­ tig e n to a c h ie v e th e ty p e o f high dose re g u la tio n observed by Vaz et a l. (2 5 ). The d iffe r e n c e s observed between IgE a n t I -DNP responses p ro ­ duced in response t o e it h e r l i g h t l y d in ltro p h e n y la te d OVA o r h e a v i­ ly d in itr o p h e n y la te d OVA a re in t e r e s t in g . Immunogenic doses o f DNP0 tj-OVA induced a tr a n s ie n t IgE a n t i -DNP response and th e t i t e r s were in v e rs e ly r e la te d t o dose w h ile immunogenic doses o f DNPg^-OVA induced a high and p e r s is te n t response. T h is d if f e r e n c e between th e tr a n s ie n t and p e r s is te n t n a tu re o f th e responses is p ro b a b ly b e st e x p la in e d on th e b a s is o f s tu d ie s by K laus and M it c h e ll ( 69 ) In which th e y showed a d i s t i n c t d iffe r e n c e In th e m etabolism o f DNPg-BSA and DNPgQ-BSA. DNPgQ-BSA p e r s is t e d ,. In v iv o , f o r long p e rio d s o f tim e , was re ta in e d m a in ly in th e spleen and l i v e r and was e f f i c i e n t l y ta k en up by macrophages, in v i t r o . 67 b u t degraded v e ry sllowHy. ONPg-BSAs on th e o th e r hand was e x c re te d r a p id ly , was more b ro a d ly d is t r ib u t e d in v a rio u s organs and was not ta k e n up as e f f i c i e n t l y by macrophages as DNPg0-BSA. Thus, i t is th o u g ht th a t perhaps th e IgE responses produced as a r e s u lt o f im­ m u n iz a tio n w ith DNPq not r e ta in e d jj-OVA a re tr a n s ie n t because t h is a n tig e n is In v iv o f o r v e ry long and th e r e fo r e is not a v a ila b le t o p ro v id e a c o n s ta n t s tim u lu s f o r IgE B c e l l s . An a d d itio n a l e x p la n a tio n , th e fo rm a tio n o f suppressor T c e l l s , may be c o n s id e re d . Recent work by Is h iz a k a and Adachi (2 2 ) showed t h a t th e Jji v i t r o c u lt u r e o f s p le n ic lym phocytes, w ith a n tig e n , th e absence o f macrophages, re s u lte d in In th e fo rm a tio n o f T sup­ p re s s o r c e l l s f o r IgE and IgG a n ti-h a p te n responses whereas c u l­ t u r in g on a n tig e n b e a rin g macrophages re s u lte d T h e lp e r c e l l s . in th e fo rm a tio n o f Thus, suppressor T c e l l s may be-form ed as a r e s u lt o f im m unization w ith DNP0 ^g-OVA b u t not w ith DNPg^-OVA because o f th e d iffe r e n c e s In th e e f f e c t on macrophages between th e two con­ ju g a te s . One o th e r f a c t o r th a t m ight be in flu e n c in g th e responses to th e DNP-c o n ju g a te s may be r e la t e d t o th e d iffe r e n c e s ses o f a n tib o d y induced by th ese a n tig e n s . in th e o th e r c la s ­ Klaus and Cross (7 0 ) showed th a t DNPg-BSA e l i c i t e d m a in ly IgG a n tib o d y and immunological memory w h ile DNPg0 -BSA Induced a p rim ary IgM response, l i t t l e IgG 68 a n tib o d y and poor memory. As mentioned above, a n t ig e n - s p e c if ic enhanced IgE fo rm a tio n in th e r a b b it (3 1 ) and a n t ig e n - s p e c if ic suppressed IgE fo rm a tio n in th e r a t ( 2 6 ) . c a r e fu l IgM IgG However, one must be in a p p ly in g o b s e rv a tio n s on IgE fo rm a tio n in one species to a n o th e r ( 4 ) . A ls o th e r e g u la to ry e f f e c t o f p a s s iv e ly a d m in is te re d IgG a n tib o d y on IgE fo rm a tio n in m ice has been shown t o be d i f f e r ­ e n t from th a t observed in th e r a t ( 7 1 ) . f a c to r s I t may be t h a t a l l o f these in flu e n c e th e IgE responses t o th e d i f f e r e n t DNP-OVA c o n ju ­ g a te s ; however f u r t h e r work Is r e q u ire d . The dichotom y In a n ti-h a p te n and a n t i - c a r r i e r observed by o th e rs (4 2 , 58) was c o n firm e d . IgE responses Mice Immunized w ith DNP0 i 5 -OVA produced high and p e r s is te n t a n t I -OVA IgE responses. It may appear th a t t h is response c o n tr a d ic ts th e e x p la n a tio n advanced above f o r th e tr a n s ie n t n a tu re o f th e a n t I -DNP response because i t im p lie s th a t a n tig e n macrophages. is re ta in e d j_n v iv o and p ro b ab ly processed by However, I t seems p ro b a b le th a t a t such a low d e n s ity o f hapten c o n ju g a tio n , th e re a re many OVA m olecules which do not c o n ta in any DNP groups and hence would be processed as " n a tiv e " alum p r e c ip it a t e d OVA and produce a t y p ic a l o f DNP24~0VA t o induce an anti-O V A IgE response. The f a i l u r e IgE response can b e s t be ex­ p la in e d by a masking o f o r a c o n fo rm a tio n a l change in th e n a tiv e OVA d e te rm in a n ts . 69 The la c k o f a d e te c ta b le PCA a n tib o d y response in m ice I immu­ n iz e d w ith OVA and B_0 p e rtu s s is v a c c in e was s u r p r is in g because o f p re v io u s work In which t h is a d ju v a n t fa v o re d th e p ro d u c tio n o f IgE (4 ). However, 1$, p e rtu s s is vac c in es a r e a p p a re n tly u n r e lia b le fo r t h i s purpose and o n ly c e r t a in p re p a ra tio n s a re a b le t o induce IgE fo rm a tio n t o s p e c if ic a n tig e n s (4 ). The in d u c tio n o f IgE responses w ith o u t a d ju v a n t, by m u lt ip le in je c tio n s o f a n tig e n has not been re p o rte d p re v io u s ly f o r la b o ra ­ to r y a n im a ls . T h is Is a s ig n if ic a n t o b s e rv a tio n because i t may re p re s e n t a model f o r IgE a n tib o d y in d u c tio n th a t Is more a k in to th e means by which a l l e r g i c s in g le th is in d iv id u a ls become s e n s itiz e d than th e in je c t io n o f a low dose o f alum -adsorbed a n tig e n . regimen Induced high IgE responses. c ip itin Although I t a ls o Induced high p re ­ responses, when MRS was used, which is not observed in th e a to p ic c o n d itio n , I b e lie v e th a t I f much lower doses o f a n tig e n a r e used then th e p a tte r n o f a n tib o d y responses w ith re g ard to im­ m unoglobulin c la s s would be a lt e r e d such th a t th e p r e c i p i t i n r e ­ sponse would be c o n s id e ra b ly d im in ish ed and th e IgE response en­ hanced. I s p e c u la te t h a t , In o rd e r t o induce IgE a n tib o d y fo rm a tio n , re p ea te d s tim u la tio n o f th e c e l l s T o r B o r b o th ) is r e q u ire d . In v o lv e d in IgE fo rm a tio n ( e it h e r T h is Is so th a t a c e r t a in minimum 70 number o f IgE producing c e l l s a r e formed In o rd e r t o a t t a i n high enough serum le v e ls o f IgE a n tib o d y th a t can be d e te c te d by the PCA a s s a y . T h is could be a ch ie v ed e it h e r by g iv in g alum -adsorbed a n tig e n o r m u lt ip le In je c tio n s o f a n tig e n s . Macrophage p ro cessin g has been shown to be im p o rtan t In the fo rm a tio n o f h e lp e r T c e l l s f o r IgE responses ( 2 2 ) . A lthough th e mechanism by which alum -adsorbed a n tig e n s tim u la te s IgE fo rm a tio n is not known, Tada (4 ) s p e c u la te s th a t sm all doses o f alum -adsorbed a n tig e n a r e p h ag o cytized by macrophages and re le a s e d ly In an extrem e­ Immunogenic form to s tim u la te c e r t a in p o p u la tio n s o f T o r B c e lls . Presum ably, alum -adsorbed a n tig e n s a re taken up v e ry e f ­ f i c i e n t l y by macrophages. It Is not known to what e x te n t s o lu b le a n tig e n s , such as those used in m u lt ip le in je c t io n s , in comparison t o alum -adsorbed a n tig e n , a re processed by macrophages. I t may be th a t by g iv in g re p ea te d In je c tio n s o f s o lu b le a n tig e n th e r e q u ir e ­ ment f o r macrophage Involvem ent is overcome o r a l t e r n a t i v e l y , th a t macrophage pro cessin g o f s o lu b le a n tig e n is s tim u la te d by t h is r e g i ­ men. From th e s tu d ie s on th e e f f e c t o f m u lt ip le , h ig h doses o f an­ t ig e n , g iv e n IV on days 3 , 5 , and 7 a f t e r p r io r im m unization w ith 10 /Ltg OVA + I mg alu m , r e s u lt s IP I t can be concluded th a t t h i s tre a tm e n t in th e s e le c t iv e suppression o f IgE responses t o OVA and 71 t h a t t h is e f f e c t can be tr a n s fe r r e d t o normal anim als by s p le n ic c e lls . W h ile th e s e s tu d ie s were in p ro g re s s , o th e r w orkers re p o rte d t h a t a d m in is tr a tio n o f 3 , 100 jug, IV In je c tio n s o f urea d e n atu rated OVA (UD-QVA) g iv e n on days 3» 5 , and 7 a f t e r im m unization w ith a low dose o f a lu m -p r e c ip ita te d OVA, suppressed anti-O V A IgE and IgG responses and r e s u lte d in th e fo rm a tio n o f suppressor T c e l l s (3 9 ). T h e ir system was unique in th a t UD-OVA c o n ta in ed o n ly th e T - c e ll s tim u la tin g d e te rm in a n ts o f n a tiv e OVA; th e n a tiv e OVA B- c e l l stim u la t i n g d e te rm in a n ts w ere lo s t by th e urea tre a tm e n t ( 7 3 ) . In l i g h t o f th e s e s tu d ie s . 3, I t seems re as o n a b le t o assume th a t 100 #zg, IV in je c tio n s o f n a tiv e OVA a ls o induces th e fo rm a tio n o f suppressor T c e l l s , however th e evidence f o r t h is Is s t i l l in ­ d i r e c t , supported m a in ly by th e f a c t t h a t suppression can be tr a n s ­ f e r r e d by s p le n ic c e l l s . One main d if f e r e n c e between th e UD-OVA and th e n a tiv e OVA used in my s tu d ie s OVA. is th e presence o f B c e l l d e te rm in a n ts on th e n a tiv e For t h is reaso n , o th e r a l t e r n a t i v e hypotheses t o e x p la in th e desens 111z a t i on observed in my system m ight be c o n s id e re d . One such h y p o th e s is Is th a t th e m u lt ip le , high dose in je c tio n s o f s o lu b le a n tig e n Induce a h ig h a f f i n i t y IgG a n tib o d y which then e x e r ts a s u p p re s s iv e e f f e c t on th e IgE response. I t has been r e ­ 72 p o rte d th a t p a s s iv e ly a d m in is te re d , a n t ig e n - s p e c if ic IgG a n tib o d y from hyperimmune a n im als w i l l e x e r t a s u p p ressive e f f e c t on IgE responses t o th e same a n tig e n (7 1 )« The e f f e c t was t r a n s ie n t how­ e v e r , and o n ly la s te d u n t i l th e tr a n s fe r r e d a n tib o d y had been c a t a b p llz e d . A ls o , th e suppression was most e f f e c t i v e when th e pas­ s iv e a n tib o d y was tr a n s fe r r e d w ith in 2k hr o f a c t iv e im m unization. The p a s s iv e ly a d m in is te re d a n tib o d y could not te rm in a te an e x is tin g IgE response o r suppress a secondary IgE response. These o b s e rv a tio n s a r e in c o n s is te n t w ith th e events seen when m ice a r e d e s e n s itiz e d w ith m u lt ip le In je c tio n s o f n a tiv e OVA, be­ cause both a p r e - e x is t in g and th e secondary IgE response were shown t o be suppressed by th e tre a tm e n t. lenge o f a n tig e n A ls o , when a secondary c h a l­ Is g iv e n t o s e n s itiz e d a n im a ls , high le v e ls o f IgE a n tib o d y a r e m a in ta in e d in th e presence o f e x tre m e ly h ig h le v e ls o f PHA a n tib o d y . The fo re g o in g argues a g a in s t th e h y p o th e s is th a t th e observed suppression o f IgE responses by a d m in is tr a tio n o f m u ltip le IV In je c tio n s o f a n tig e n is due t o an IgG a n tib o d y m ediated mecha­ nism . A nother h yp o th e s is th a t m ight be considered Is th e s e le c t iv e in d u c tio n o f IgE B c e l l to le r a n c e . However, In th e case o f p ro te in a n tig e n s , th e in d u c tio n o f to le ra n c e in p r e s e n s itiz e d B c e l l s has proved t o be d i f f i c u l t t o accom plish ( 7 * 0 . Some re c e n t s tu d ie s 73 have shown th a t to le ra n c e In IgE B c e l l s could be a c h ie v e d , even a f t e r p r i o r s e n s i t i z a t i o n , by DNP coupled to non-immunogenic c a r ­ r ie r s ( 6 2 , 7 5 ) , and th a t th e dose o f to le ro g e n re q u ire d f o r IgE to le r a n c e in d u c tio n was le s s than t h a t re q u ire d f o r in d u c tio n ( 7 5 ) . It IgG to le ra n c e is not known w hether t h is dosage e f f e c t a p p lie s to th e in d u c tio n o f IgE B c e l l to le r a n c e to p r o te in a n tig e n s , . A lth o u g h th e in d u c tio n o f B c e l l to le ra n c e cannot be ru le d o u t, th e f a c t th a t th e suppression o f Ig E , which Is m u lt ip le , induced by IV in je c tio n s o f a n tig e n can be tr a n s fe r r e d t o o th e r a n i ­ mals by s p le n ic c e l l s makes t h is mechanism as th e o n ly e x p la n a tio n o f d e s e n s itiz a t io n h ig h ly u n lik e ly and suggests v e ry s tr o n g ly th e presence o f a suppressor c e l l . SUM MARY The r e s u lt s o f t h i s th e s is can be summarized as fo llo w s : 1) The suppression o f IgE a n tib o d y responses by neonatal Iy i n i t i a t e d a n t i - # tre a tm e n t showed th a t Ig E -fo rm in g c e l l s a r e de­ r iv e d from th e same p re c u rs o rs ! c e l ! as a r e th e c e l l s form ing o th e r c la s s e s o f Im m unoglobulins. 2) V a rio u s ways o f inducing and r e g u la tin g B a lb /c m ice were compared. IgE responses in Im m unization w ith alum -adsorbed OVA o ver a w ide dosage range Induced a h ig h and p e r s is te n t IgE response. F a i lu r e t o show a tr a n s ie n t IgE response t o high doses o f a n tig e n may be r e la t e d t o th e I n a b i l i t y o f I mg alum to c o m p le te ly re a c t w ith an excess o f p r o t e in . Im m unization w ith alum -adsorbed DMP- o v a Ibumln c o n ju g ates produced 2 d i f f e r e n t p a tte rn s o f IgE responses r e la t e d t o th e hapten d e n s ity . T h is was thought t o be r e la te d to th e way In which th e s e c o n ju g ates may be m e ta b o liz e d . Im m unization w ith one in je c t io n o f OVA w ith B. p e rtu s s is f a i l e d t o induce IgE resp o nses. A new means o f Inducing IgE responses by m u lt ip le IP in je c tio n s o f a n tig e n was shown. A ls o , tre a tm e n t w ith n e o n a ta lIy I n i t i a t e d m u lt ip le in je c tio n s o f MRS suppressed subsequent immune responses t o Im m unization w ith alum -adsorbed OVA. 3) The s e le c t iv e suppression o f IgE responses by 3 , IV in je c ­ tio n s o f high doses o f s o lu b le a n tig e n g iv en on days 3 , 5 , and 7 a f t e r p r i o r s e n s it iz a t io n w ith 10 fig OVA + I mg alum was shown to 75 be due, a t th e s p le e n . le a s t In p a r t to th e g e n e ra tio n o f suppressor c e l l s In Both a n tib o d y t i t e r s and th e number o f IgE producing c e l l s were reduced to u n d e te c ta b le le v e ls by t h is tre a tm e n t. T h is phenomenon was a n tig e n s p e c if ic and v e ry dependent on th e ro u te o f a d m in is tr a tio n . Whereas 100 (ig o f s o lu b le a n tig e n was v e ry sup­ p re s s iv e when g iv e n IV , th e same o r 1 0 -fo ld h ig h e r doses were o n ly s l i g h t l y s u p p re s s iv e when g iv en IP , g e n ic dose, 10 jug, g iv en IV , However, an o p tim a lly Immuno­ Induced s ig n if ic a n t s u p p res s io n . ondary responses were a ls o a f f e c t e d by t h is tre a tm e n t. Sec­ LITERATURE CITED 1. I SHI ZAKA, K . : Chem istry and b io lo g y o f Immunoglobulin E„ in SELA The A n tig e n s , V o l„ I , p„ 479 (Academic P re s s , New Y ork, 1 9 7 3 ). 2. I SN I ZAKA, T . and ISHIZAKA, K .: P ro g e A lle r g y J 9 : 60 (1 9 7 5 ), 3. PATTERSON, R. and SPARKS, D, B .: The passive t r a n s f e r to n o r­ mal dogs o f s k in r e a c t i v i t y , asthma and a n a p h y la x is from a dog w ith spontaneous ragweed p o lle n h y p e r s e n s it iv it y . J e lmmun, 88: B io lo g y o f Immunoglobulin Ee 262 ( 1962) . — 4. TADA, T . : R e g u la tio n o f r e a g ln lc a n tib o d y fo rm a tio n in a n i ­ m a ls . P rog. A lle r g y 1 9 : 122 (1 9 7 5 ). 5. LYNCH, N. R. and TURNER, K. J . : Time course o f hom ocytotropic and p a s s iv e hemaggIu t I n a t I n g a n tib o d y s y n th e s is in th e marsu­ p i a l S e to n lx brachyurus (th e q u o k k a ). I n t e Archs A lle r g y a p p l. Immun. 4 7 : 933 (1 9 7 4 ). 6. KANYERZI , B .; JATON, J . C. and BLOCH, K. J . : Human and r a t 7 E S e ro lo g ic e vid en ce o f homology, J . Immune 106; 1411 (1 9 7 1 ). 7. HALLIWELL, R .E .W .; SCHWARTZMAN, R. M. and ROCKEY, J . H .: A n t i ­ g e n ic r e la t io n s h ip between human IgE and c a n in e Ig E e C l l n e Exp. lmmun. JJ): 399 (1 9 7 2 ). 8. GOLLAPUDI, V .S .S . and KIND, L . S .: Enhancement o f r e a g ln lc an ­ tib o d y fo rm a tio n In th e mouse by co n ca n a v ailn A . I n t e Archs A l ­ le r g y a p p l. lmmun, 4 8 : 94 (1 9 7 5 ). 9. KIND, L . S. and MACEDQ-SOBRINHO, Be : R e a g in ic a n tib o d y form a­ t i o n In mice in je c te d w ith r a b b it a n t I -mouse thym ocyte serum. I n t e Archs A lle r g y a p p l. lmmun, 4*»: 780 (1 9 7 3 ). 10. CLAMAN, H. N. and CHAPERON, E. A . : Immunological complementa­ t i o n between thymus and marrow c e l l s . A model f o r th e tw o -c e ll th e o ry o f immunocompetence. T ra n s p la n tn . Rev. 92 (1 9 6 9 ). 11. UNANUE, E. R .: R e g u la to ry r o l e o f macrpphages In a n tig e n ic s t im u la t io n . Adve lmmun. Jj?: 95 (1 9 7 2 ). 77 12. TADA, T . and OKUMURA, K .: R e g u la tio n o f homocyt o t ro p Ic a n t i ­ body fo rm a tio n in th e r a t . V . C e ll c o o p e ra tio n in th e a n t i ­ hapten hom ocytotro p Ic a n tib o d y response. J . Immun0 107: 1137 (1 9 7 1 ). 13. HAMAOKA, T . ; KATZ, D. H. and BENACERRAF, B .: H a p te n -s p e c ific IgE a n tib o d y responses in m ice . I I . C o o p e ra tiv e in te r a c tio n s between a d o p tiv e ly t r a n s fe r r e d T and B lymphocytes in th e de­ velopm ent o f IgE response. J . Exp. Med. J 3 8 : 538 (1 9 7 3 ). 14. OKUDAI RA, H. and I SHI ZAKA, K .: R e a g ln ic a n tib o d y fo rm a tio n in th e mouse. I I I . C o lla b o r a tio n between h a p te n -s p e c ific memory c e l l s and c a r r i e r - s p e c i f i c h e lp e r c e l l s f o r secondary a n t i ­ hapten a n tib o d y fo rm a tio n . J . Immun. I l l : 1420 (1 9 7 3 ). 15. OKUDAI RA, H. and I SHI ZAKA, K .: R e a g in ic a n tib o d y fo rm a tio n in th e mouse. IV . A d o p tiv e a n t I -h a p te n IgE a n tib o d y response in ir r a d ia t e d r e c ip ie n ts o f h apten-prim ed c e l l s and c a r r i e r s p e c if ic c e l l s . J . Immun. 113: 563 (1 9 7 4 ). 16. KlSHI M0T0, T . and I SHI ZAKA, K .: R e g u la tio n o f a n tib o d y r e ­ sponse in v i t r o . I I I . R ole o f h a p te n -s p e c ific memory c e lls and c a r r i e r - s p e c i f i c h e lp e r c e l l s on th e d is t r i b u t i o n o f an­ t i- h a p t e n a n tib o d y in IgG, IgM and IgE c la s s e s . J . Immun, 109: 612 (1972). 17. 0KUMURA, K. and TADA, T . : R e g u la tio n o f hom ocytotro p ic a n t i ­ body fo rm a tio n in th e r a t . I I I . E ffe c t o f thymectomy and splen ectom y. J . Immun. 106: 1019 (1 9 7 1 ). 18. TADA, T . ; 0KUMURA, K. and TAN I GUCHI , M0 : C e l lu la r b a sis o f r e a g ln ic a n tib o d y fo rm a tio n in th e r a t ; In I SHI ZAKA and DAYTON The b io lo g ic a l r o le o f immunoglobulin E system , p . 89 (U .S . Government P r in t in g O f f i c e , W ashington 1 9 7 4 ). 19. MICHAEL, J . G. and BERNSTEIN, I . L . : g ln ic a n tib o d y fo rm a tio n in m ice . J , 20. SCHNELLER, R. L . : R e g u la to ry e f f e c t s o f th e thymus on th e IgE response o f m ice. M a s te r's t h e s is , Montana S ta te U n iv e r s ity (1 9 7 4 ). Thymus dependence o f rea Immun. I l l : I 600 (1 9 7 3 ). 78 21. KATZ, D. H .; HAMAOKA, T . ; NEWBERGER, P. E. and BENACERRAF, B .: H a p te n -s p e c ific IgE a n tib o d y responses in m ice . IV . Evidence f o r d i s t i n c t i v e s e n s i t i v i t i e s o f IgE and IgG B lymphocytes to th e r e g u la to r y in flu e n c e s o f T c e l l s . J . Immun. 113: 974 (1 9 7 4 ). 22. I SHI ZAKA, K. and ADACHl, T . : G en era tio n o f s p e c if ic h e lp e r c e l l s and suppressor c e l l s in v i t r o f o r th e IgE and IgG a n t i ­ body responses, J . Immun. 117: 40 (1 9 7 6 ). 23. I SHIZAKA, K. and I SHI ZAKA, T . : R ole o f IgE and IgG a n tib o d ie s in r e a g in ic h y p e r s e n s it iv it y in th e r e s p ir a to r y t r a c t , in : AUSTEN and LICHTENSTEIN Asthma: p h y s io lo g y , 5mmunopharmacology, and tr e a tm e n t, p . 55 (Academic P re s s , New York 1 9 7 3 ). 24. OGI LVI E, B. M. and JONES, V . E .: N lp p o s tro n q y lu s b r a s i l lens I s . A re v ie w o f immunity and th e h o s t/p a r a s ite r e la t io n s h ip In th e r a t . E x p tI . Paras i t o l . 2*1: 138 (1 9 7 1 ), 25. VAZ, E. M .; VAZ, N. M. and LEVINE, B .: P e r s is te n t fo rm a tio n o f re a g ln s In m ice in je c te d w ith low doses o f ovalbum in. Im­ munology 2J_: 11 (1 9 7 1 ). 26. TADA, T . and OKUMURA, K .: R e g u la tio n o f hom ocytotropic a n t i ­ body fo rm a tio n In th e r a t . I . Feed-back r e g u la tio n by p a s s iv e ­ ly a d m in is te re d a n tib o d y . J , Immun. J 0 6 : 1002 (1 9 7 1 ). 27. OKUMURA, K .; TADA, T . and OCHIA I , T . : E ffe c t o f a n t I thymo­ c y te serum on r e a g in ic a n tib o d y fo rm a tio n in th e r a t . Immu­ nology 2 6 : 257 (1 9 7 4 ). 28. TADA, T . ; TANIGUCHi, M. and OKUMURA, K .: R e g u la tio n o f homo­ c y t o tr o p ic a n tib o d y fo rm a tio n in th e r a t . I I . E f f e c t o f x I r r a d l a t Io n . J . Immun. 106; 1012 (1 9 7 1 ). 29. OKUMURA, K. and TADA, T . : R e g u la tio n o f hom ocytotropic a n t i ­ body fo rm a tio n in th e r a t . V I . In h ib it o r y e f f e c t o f thymocytes on th e hom ocytotropic a n tib o d y response. J . Immun. 107: 1682 0 9 7 1 ). 30. STRANNEGARD, 0 . and BELIN, L . : Suppression o f re a g ln synthe­ s is in r a b b its by p a s s iv e ly a d m in is te re d a n tib o d y . Immunology ! 8 : 775 (1 9 7 0 ). 79 3 Io STRANNEGARD, 0 . and BEL IN , Le : Enhancement o f re a g ln form a­ t io n in r a b b its by p a s s iv e ly a d m in is te re d 19S a n tib o d y . Im­ munology 20: 4 2 7 (1 9 7 1 )o 32. LEVINE, Be B, and VAZ, N„ M .: E ffe c t o f com binations o f In bred s t r a i n , a n tig e n , and a n tig e n dose on immune re sp o n s iv e ­ ness and re a g ln p ro d u c tio n , A p o te n tia l mouse model f o r Immune a sp e c ts o f human a to p ic a l l e r g y . I n t . Archs A lle r g y a p p l. Immun. 3^: 156 (1 9 7 0 ), 33. McDEVITT, H. 0 . and BENACERRAF, B .: G en etic c o n tr o l o f s p e c if ic Immune responses. Adv. Immunol, j_ [: 31 (1 9 6 9 ). 34. KIND, L . S. and MALLOY, W. F , : Development o f r e a g in lc a n t i b o d y -fo rm in g c e l l s in th e s p leen and bone-marrow o f immunized m ice . J . Immun. 112: 1609 (1 9 7 4 ). 35. GOLLAPUDI, V .S .S . and KIND, L . S .: Phenotypic c o r r e c tio n o f low re a g ln p ro d u c tio n : A g e n e tic d e fe c t in th e SJL mouse, J . Immun. 114 : 906 (1 9 7 5 ). 36. WATANABE, N .; K0JIMA, S. and OVARY, Z , : Suppression o f IgE a n tib o d y p ro d u c tio n In SJL m ice . I . N o n s p e c ific suppressor T c e l l s . J . Exp, Med. j 4 £ : 833 (1 9 7 6 ). 37. MAIA, L .C .S .; VAZ, N. M. and VAZ, E. M .: E ffe c t o f s o lu b le an­ t ig e n on IgE responses in th e mouse. I n t . Archs A lle r g y a p p l. Immun. 4 6 : 339 (1 9 7 4 ). 38. BACH, M. K. and BRASHLER, J . R .: IgE a n tib o d y -s p e c ific ab­ r o g a tio n o f an e s ta b lis h e d immune response in m ice by modi­ f i e d a n tig e n s . J . Immun. 114 : 1799 (1 9 7 5 ). 39. TAKATSU, K. and ISHIZAKA, K .: R e a g in lc a n tib o d y fo rm a tio n in th e mouse. V I I . In d u c tio n o f suppressor T c e l l s f o r IgE and . IgG a n tib o d y responses. J . Immun. 116: 1257 (1 9 7 6 ). 4b. O G ILVIE, B. M .: R e a g ln -I Ik e a n tib o d ie s in r a ts in fe c te d w ith th e nematode p a r a s ite N lp p o s tro n q y lu s bras 1 1 ie n s is . Immunology j 2 : 113 (1 9 6 7 ). 41. EISEN, H. N .; BELMAN, S . and CARSTEN, M. E .: The In te r a c tio n o f d I n itro b e n z e n e d e r iv a tiv e s w ith bovine serum a lb u m in . J . Amer. Chem See. 75: 4853 (1 9 5 3 ). 80 42. t SHIZAKAy K. and OKURAI RA, H .: R e a g in ic a n tib o d y fo rm a tio n in th e mouse. I I . Enhancement and suppression o f a n ti-h a p te n a n tib o d y fo rm a tio n by p rim in g w ith c a r r i e r . J . !mmun. 110; 106? (1 9 7 3 ). 43. IVERSON, G. M .: Assay methods f o r a n tig e n -m e d ia te d c e l l co­ o p e r a tio n ; in WEIR Handbook o f e xp e rim e n ta l immunology, V o l. 2 , p . 2 9 .3 (B la c k w e ll S c i e n t i f i c P u b lic a tio n s , O xford 1 9 7 3 ). 44. MOTA, I . and WONG, D .: Homologous and h e te ro lo g o u s pa ss iv e cutaneous a n a p h y la c tic a c t i v i t y o f mouse a n tis e r a d u rin g th e course o f im m unization. L i f e S c i. 8 : 813 (1 9 6 9 ). 45. OVARY, Z . ; CAIZZA, S. Se and K0JIMA, S .: PCA re a c tio n s w ith mouse a n tib o d ie s in m ice and r a t s . I n t . Archs A lle r g y a p p l. Immun. 4 8 : 16 (1 9 7 5 ). 46. KIND, L . S. and MACED0-S0BRINH0, B .: H eterologous a d o p tiv e cutaneous a n a p h y la x is . A method f o r d e te c tin g r e a g in lc a n t i ­ body fo rm a tio n by c e l l s o f th e mouse. J . Immun. I l l : 638 (1 9 7 3 ). 47. JOHNSON, H. M .; BRENNER, K. and HALL, H. E .: The use o f a w a te r s o lu b le c a rb o d llm ld e as a c o u p lin g re ag e n t in th e pas­ s iv e h e m a g g lu tin a tio n t e s t . J . I mmun. <17: 791 (1 9 6 6 ), 48. JOHNSON, H. M .; SMITH, B. G. and HALL, H. E .: C arb o d ilm id e h e m a g g lu tin a tio n — a study o f some o f th e v a r ia b le s o f th e coup­ lin g r e a c tio n . I n t . Archs A lle r g y 2 3 : 511 (1 9 6 8 ). 49. SWEET, G. H. and WELBORN, F . L . : Use o f chromium c h lo r id e as th e c o u p lin g agent in a m o d ifie d plaque assay c e l l s producing a n t i - p r o t e i n a n tib o d y . J . Immun. 106: 1407 (1 9 7 1 ). 50. MANNING, D. D. and JUTI LA, J . W .: Immunosuppression o f con­ g e n i t a l l y athym lc (nude) mice w ith h etero lo g o u s anti-im m uno­ g lo b u lin heavy c h a in a n t is e r a . C e l l . Immun. J 4 : 453 (1 9 7 4 ). 51. JACOBSON, R. H. and REED, N. D .: The Immune response o f con­ g e n i t a l l y athym lc (nude) m ice t o th e In t e s t i n a l nematode N lp p o s tro n g y lu s b r a s i I lens i s . P ro c . Soc. Exp. B i o l . Med. 147: 667 (1 9 7 4 ). 81 52. ISHiZAKA, K .; I SHIZAKA, T . and ARBESMAN, C. E .: In d u c tio n o f p a s s iv e cutaneous a n a p h y la x is In monkeys by human yE a n tib o d y . J . A lle r g y 1 2 : 25^ (1 9 6 7 ). 53. PROUVOST-DAMON, A ; BINAGHI, R . ; ROCHAS, S. and BOUSSAC-ARON, Y. Immunochemical I d e n t i f i c a t i o n o f mouse Ig E . Immunology 23: 48* (1 9 7 2 ). — 54. KONI G, W .; OKUDAIRA, H. and I SHIZAKA, K .: S p e c ific b in d in g o f mouse IgE w ith r a t mast c e l l s . J . Immun. 112: 1652 (1 9 7 4 ). 55. CLAUSEN, C. R .; MUNOZ, J . and BERGMAN, R0 K .: R e a g ln lc -ty p e o f a n tib o d y In m ice s tim u la te d by e x tr a c ts o f B o r d e te lla p e r­ t u s s is . J . Immun. 103: 768 (1 9 6 9 ). 56. JARRETT, E . E . E . ; ORR, T .S .C . and RILEY, P .: In h i b i t i o n o f a l ­ l e r g i c re a c tio n s due t o c o m p e titio n f o r mast c e l l s e n s it iz a ­ t i o n s it e s by two re a g in s . C l i n . Exp. Immunol. 9 : 585 (1 9 7 1 ). 57. MANNING, D. D .: Heavy c h a in is o ty p e suppression: A re v ie w o f th e Immunosuppressive e f f e c t s o f h etero lo g o u s a n t i - t g heavy c h a in a n t i s e r a . J . R e tic u lo e n d o th e l. Soc. JJEI: 63 (1 9 7 5 ). 58. KISHIMOTO, T . and ISHIZAKA, K .: R e g u la tio n o f a n tib o d y r e ­ sponse j_n v i t r o . IV , Heavy c h a in a n tig e n ic d e te rm in a n ts on h a p te n -s p e c ific memory c e l l s . J . Immun. 109: 1163 (1 9 7 2 ). 59. MANNING, D. D .; MANNING, J . K. and REED, N. D .: Suppression o f r e a g in ic a n tib o d y (Ig E ) fo rm a tio n in m ice by tre a tm e n t w ith a n ti-jit a n tis e ru m . J . Exp. Med. 144 : 288 (1 9 7 6 ). 60. DWYER, J . M .; ROSENBAUM, J . T . and LEWIS, S .: The e f f e c t o f a n t i -4 suppression o f 7 M and 76 on th e p ro d u c tio n o f 7 E. J . Exp. Med. 143: 781 (1 9 7 6 ). 61. STREJAN, G. H. and MARSH, D. G .: H a p te n -c a rr ie r re la tio n s h ip s in th e p ro d u c tio n o f r a t h cm ocytotropic a n tib o d ie s . J . Immun. 1 07 : 306 (1 9 7 1 ). 62. LEE, W. Y . and SEHON, A . H .: Suppression o f r e a g in ic a n tib o d y fo r m a tio n . I . In d u c tio n o f h a p te n -s p e c ific to le r a n c e . J . Immun. 1 1 4 : 829 (1 9 7 5 ). 82 63« LEHRER9 S. B, and VAUGHAN, J . H .: P ro p e rtie s o f mouse homoc y t o tr o p lc and h e te r o c y to tr o p ic a n tib o d ie s . J . A lle r g y C lin . Immunol. £ 7 : 422 (1 9 7 6 ). 64. TADA9 T . ; OKUMURA, K .; PLATTEAU9 B .; BECKERS, A . and BAZlN9 H .: H a l f - l i v e s o f two hcm ocytotropfc a n tib o d ie s in c ir c u l a t i o n and in th e s k in . I n t . Archs A lle r g y a p p l. lmmun. 4 8 : 116 (1 9 7 5 ). 65. I SHIZAKA9 T . ; KONIG9 W .; KURATA9 M .; MAUSER9 L . and ISHIZAKA9 K .: Immunologic p r o p e r tie s o f mast c e l l s from r a ts In fe c te d w ith N ip p o s tro n q y lu s b r a s l l l e n s l s . J . lmmun. 1 15: 1078 (1 9 7 5 ). 66. SADUN9 E. H .: H om ocytotroplc a n tib o d y response t o p a r a s it ic in f e c t io n s ; In Immunity o f anim al p a r a s ite s , p . 97 (Academic P re s s 9 New Y o rk 9 1 9 7 2 ). 67. OG ILVIE9 B. M. and LOVE9 R. J . : C ooperation between a n tib o d ie s and c e l l s In Immunity to a Nematode p a r a s it e . T ra n s p la n tn . Rev. 1 2 : 147 (1 9 7 4 ). 68. PROSS9 H. F . and EiDINGER9 D .: A n tig e n ic c o m p e titio n : A r e ­ v ie w o f n o n s p e c ific a n t lg e n - Induced s u p p res s io n . A dv. lmmun. 18: 133 (1 9 7 4 ). 69. HERBERT, W. J . : ® 'M in e r a l- o t I a d ju v a n ts and th e Im m unization o f la b o ra to ry a n im a ls ; In WEIR Handbook o f e x p e rim e n ta l Im­ munology, V o l. 3 , p . A 2 . 10 (B la c k w e ll S c i e n t i f i c P u b lic a tio n s , O xford 1 9 7 3 ). 70. KLAUS9 G .G .B . and MITCHELL, G. F . : The In flu e n c e o f e p ito p e d e n s ity on th e immunological p r o p e r tie s o f h a p te n -p ro te in con. J u g a te s . I I . The In v iv o and In v i t r o m etabolism o f h e a v ily and l i g h t l y co n ju g ated p r o t e in . Incnun. 27: 699 (1 9 7 4 ). 71. KLAUS9 G .G .B . and CROSS9 A . M .: The In flu e n c e o f e p ito p e den­ s i t y on th e Immunological p r o p e r tie s o f h a p te n -p ro te in c o n ju ­ g a te s . I . C h a r a c te r is tic s o f th e immune response t o h a p te n coupled albumen w ith v a ry in g e p ito p e d e n s ity . C e l l , lmmun. 14: 226 (1 9 7 4 ). 7 2. I SHI ZAKA9 K. and OKUDAI RA 9 H . z R e a g ln lc a n tib o d y fo rm a tio n In th e mouse. I . A n tI body-m ediated suppression o f r e a g in ic a n t i ­ body fo rm a tio n . J . lmmun, 109: 84 (1 9 7 2 ). 83 73. TAKATSU, K. and I SHIZAKA, K .: R e a g in lc a n tib o d y fo rm a tio n in th e mouse. V I . Suppression o f IgE and IgG a n tib o d y responses t o ovalbum in fo llo w in g th e a d m in is tr a tio n o f h ig h dose u re a d en atu red a n tig e n . C e l l , immun. 2 0: 276 (1 9 7 5 ). 7 4. WEIGLE, W. 0 . : 1 6 ; 6 i (1 9 7 3 ). 75. KATZ, D. H .; HAMAOKA, T . and BENACERRAF, B .: In d u c tio n o f im m unological to le ra n c e In bone m arro w -d erived lymphocytes o f th e IgE a n tib o d y c la s s . F re e . N a t. Acad. S c i. U .S .A . 70: 2776 (1 9 7 3 ). Immunological unresponsiveness. Adv. Immun. 3 1762 10010986 5 D378 M3161+ eop.2 M a n n i n g , Judith Estelle (Klein) 19^0R e g u l a t i o n o f IgE anti b o d y responses in mice. DATE IS S U E D TO ..... mmm