PHGN341: Thermal Physics Exam II - April 13, 2012 NAME:
advertisement
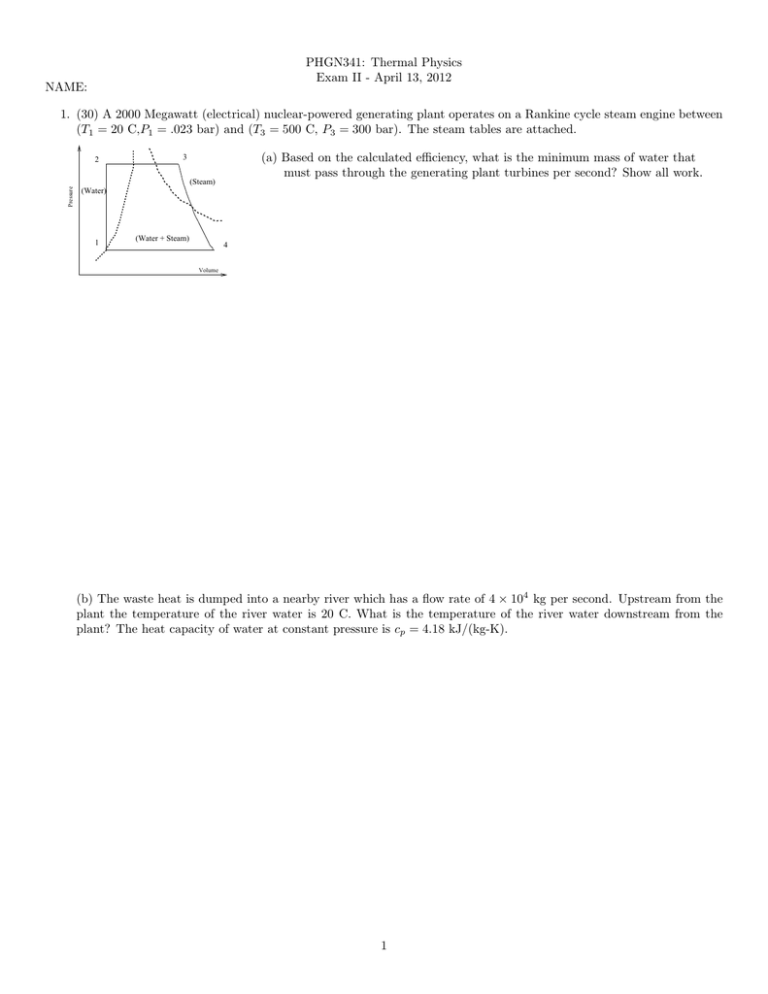
PHGN341: Thermal Physics Exam II - April 13, 2012 NAME: 1. (30) A 2000 Megawatt (electrical) nuclear-powered generating plant operates on a Rankine cycle steam engine between (T1 = 20 C,P1 = .023 bar) and (T3 = 500 C, P3 = 300 bar). The steam tables are attached. 2 (a) Based on the calculated efficiency, what is the minimum mass of water that must pass through the generating plant turbines per second? Show all work. 3 Pressure (Steam) (Water) 1 (Water + Steam) 4 Volume (b) The waste heat is dumped into a nearby river which has a flow rate of 4 × 104 kg per second. Upstream from the plant the temperature of the river water is 20 C. What is the temperature of the river water downstream from the plant? The heat capacity of water at constant pressure is cp = 4.18 kJ/(kg-K). 1 2. (30)Aluminum silicate (Al2 SiO5 ) can exist in three crystalline forms: kyanite, andalusite, and sillimanite. The thermodynamic properties for one mole of these forms are given in the table below. Substance Kyanite Andalusite Sillimanite ∆f H (kJ) -2594.29 -2590.27 -2587.76 ∆f G (kJ) -2443.88 -2442.66 -2440.99 S (J/K) 83.81 93.22 96.11 V (cm3 ) 44.09 51.53 49.90 (a) The temperature of the earth increases by about 20 C per kilometer of depth while the pressure increases by about 32 MPa per kilometer of depth. Assume that the ground level temperature is 25 C (298 K) and the pressure is 1 bar (105 Pa). Write down the equations for the temperature and pressure as a function of depth, z. (b) What is the stable form of aluminum silicate at a depth of 50 km? Show all work and give your reasoning. 2 3. (40)(a) Consider an ideal gas consisting of N indistinguishable (massless) neutrinos at temperature, T . Recall the particle-in-a-box result for the density of states in momentum space: d3 n = hV3 d3 p. Use this to calculateRthe partition function for a single neutrino by integrating the Boltzmann factor times the density of states, i.e. Z = d3 n e−βEp , where the kinetic energy is given by the ultrarelativistic relation: Ep = pc where c is the speed of light. (b) Calculate the average energy of one neutrino. Comment on your result. (c) What is the partition function for the N-neutrino gas? (Use the Sterling’s approximation result, N ! ' N N e−N .) (d) From the Helmholtz free energy, F = −kT ln Z, calculate the pressure for the neutrino gas. Comment on your result. 3 Steam Tables T (◦ C) 0 10 20 30 50 100 P (bar) 0.006 0.012 0.023 0.042 0.123 1.013 Hwater (kJ) 0 42 84 126 209 419 Hsteam (kJ) 2501 2520 2538 2556 2592 2676 Swater (kJ/K) 0 0.151 0.297 0.437 0.704 1.307 Ssteam (kJ/K) 9.156 8.901 8.667 8.453 8.076 7.355 Table 1. Thermodynamic properties of saturated water/steam for 1 kg of material. P (bar) 1.0 3.0 10 30 100 300 Property H (kJ) S(kJ/K) H (kJ) S(kJ/K) H (kJ) S(kJ/K) H (kJ) S(kJ/K) H (kJ) S(kJ/K) H (kJ) S(kJ/K) 200 2875 7.834 2866 7.312 2828 6.694 300 3074 8.216 3069 7.702 3051 7.123 2994 6.539 400 3278 8.544 3275 8.033 3264 7.465 3231 6.921 3097 6.212 2151 4.473 500 3488 8.834 3486 8.325 3479 7.762 3457 7.234 3374 6.597 3081 5.791 Table 2. Thermodynamic properties of superheated steam for 1 kg of material. 4 600 3705 9.098 3703 8.589 3698 8.029 3682 7.509 3625 6.903 3444 6.233











